William M. Mendenhall, Anthony A. Mancuso, Jessica M. Kirwan, John W. Werning, and Franklin P. Flowers
The purpose of this chapter is to discuss cutaneous carcinoma and melanoma. Uncommon cutaneous malignancies, such as angiosarcoma and dermatofibrosarcoma protuberans, will be addressed elsewhere. Most skin cancers are managed surgically. Because of the functional cosmetic deficits that may occur after surgery for skin cancers of the head and neck, the discussion will occasionally focus on lesions in this area.
Skin cancer is the most common of all malignancies. The American Cancer Society estimates that approximately two million basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) are diagnosed annually in the United States, although the precise number is unknown because they are not reported.1 The risk for carcinoma of the skin increases with sun exposure. A low incidence occurs in dark-skinned people; a corresponding increase occurs in those with a fair, ruddy, Scotch-Irish complexion. The mortality rate is about 1,800 per year, or 0.45%.
Several conditions are associated with carcinoma of the skin, including the following:
• Actinic exposure
• Ionizing radiation
• Scar (e.g., burn scar)
• Chronic draining of sinus or fistulous tract (e.g., pilonidal sinus)
• Immune disorders
• Chronic lymphocytic leukemia
• Solid organ transplant patients
• Discoid lupus erythematosus
• Chemicals
• Arsenicals (herbicides, pesticides)
• Psoralens and ultraviolet light (PUVA) treatment for psoriasis54
• Nitrates
• Tars, oils, and paraffins
• Hereditary disorders
• Xeroderma pigmentosum
• Basal cell nevus syndrome
• Albinism
• Congenital epidermolysis bullosa
Melanoma is less common than BCCs and SCCs. Jemal et al.2 estimated that approximately 68,130 melanomas will be diagnosed in the United States in 2010 and that about 8,700 deaths owing to the disease will occur.
![]() ANATOMY
ANATOMY
The epidermis is thinner in the face than in most portions of the body, measuring approximately 0.04 mm. No consistent change in the thickness of the epidermis occurs with increasing age, and no difference in skin thickness exists between men and women.
The dermis, which contains the blood and lymphatic vessels, adnexa, hair follicles, sweat glands, and sebaceous glands, is 1 to 2 mm thick; the dermis of the eyelid is thinner, ≤0.6 mm. Beneath the dermis lies the subcutaneous tissue containing the fat and the superficial fascia. No distinct transition occurs from the dermis to the subcutaneous layer.
Lymphatics
No lymphatics exist in the epidermis. A superficial capillary lymphatic plexus lies in the dermis and is without valves.3 The deep lymphatic trunks in the dermis and subcutaneous tissues have valves. The density of the capillary lymphatics has been noted to be about the same in all areas, except the sole of the foot and palm of the hand, where it is denser. Observation suggests that SCCs and melanomas occurring on the skin of the temple are particularly prone to develop lymphatic metastasis.4 During the healing of wounds, such as incisions or burns, a regeneration of lymphatic capillaries across the scar occurs, similar to the regrowth of small blood vessels.3
The first-echelon lymph nodes for carcinomas of the face and scalp are the superficial network of lymph nodes that form a ring around the top of the neck: submental (level IA), submandibular (level IB), parotid area, postauricular (mastoid), and occipital lymph nodes as well as inconstant facial lymph nodes.
![]() PATHOLOGY
PATHOLOGY
The most common carcinomas of the skin are BCC (65%), SCC (30%), or one of their variants and the adnexal carcinomas. Merkel cell carcinoma (MCC) is a rare neuroendocrine malignancy arising in the skin that was first described by Toker5 in 1972. Verrucous carcinoma, a variant of SCC, occurs most often on the foot and is rare in the head and neck area. Carcinoma in situ (CIS) occurs frequently in the head and neck. Perineural invasion (PNI) is observed in 2% to 3% of patients with BCCs and SCCs.6
Noncutaneous carcinomas (e.g., renal cell carcinoma) may metastasize to the skin and subcutaneous tissues.
Basal Cell Carcinoma
The common BCC, which arises from the basal layer of the epithelium, may have a variety of growth patterns merge into one another in the same tumor, and the different names applied to the gross and microscopic appearances have clinical implications. The morphea type (sclerosing BCC) shows little surface disease and a marked infiltrating pattern; it is an important subtype because of the higher risk for recurrence. Microcystic BCC is a histologic variant of BCC that exhibits the same natural history as the more common variety. Some lesions will have mixed BCC and SCC (basosquamous cell carcinoma or metatypical BCC).
Melanin pigment may be seen on both gross and microscopic examination of BCC.
Squamous Cell Carcinoma
SCC and its variants (i.e., verrucous carcinoma, spindle cell SCC) are similar histologically to SCC occurring in other sites. Most are well differentiated. Evans and Smith7 identified two categories of spindle cell tumors of skin: one was composed of SCC mixed with a spindle cell component; the other was predominantly spindle cells. The spindle cell component is similar in both groups; mitoses, giant cells, and epithelioid cells may be seen.
Keratoacanthoma
Keratoacanthoma is a benign tumor of the skin that grossly resembles a cystic BCC and microscopically resembles SCC or squamous papilloma. Part of the difficulty in histologic diagnosis is owing to an inadequate biopsy specimen. Ackerman8 concluded that “the diagnosis of keratoacanthoma can only be made with absolute certainty by biologic behavior in the form of eventual involution.”
Adnexal Carcinoma
Carcinomas may arise from the epithelium of the sweat glands, sebaceous glands, or hair follicles and microscopically resemble the tissue of origin. Carcinoma of the sweat gland can arise from either the eccrine or apocrine glands; however, no reliable histologic criteria exist to differentiate the origin. It is difficult to distinguish between benign tumors and malignant carcinomas of the sweat gland in the absence of metastases. The differential diagnosis includes adenocarcinoma metastatic to skin and BCCs and SCCs with an adenoid cystic growth pattern. They are frequently misdiagnosed at the time of the first biopsy. Malignant trichilemmoma arising from hair follicles is extremely rare.
Microcystic adnexal carcinoma (sclerosing sweat duct carcinoma) is a rare variant of adnexal carcinoma first described in 1982. The lesion usually presents on the upper lip or skin of the periorbital area as an indurated plaque without direct invasion of the overlying epidermis. It tends to be slow growing and locally invasive.9–11 Microcystic adnexal carcinoma exhibits a propensity for local recurrence after excision and is associated with PNI; lymph node metastases are rare. The role of radiation therapy (RT) in the treatment of this rare malignancy is undefined.
Merkel Cell Carcinoma
MCC is a small cell neuroendocrine carcinoma arising in the skin. As in neuroendocrine carcinomas arising in other primary sites, MCC produces a neuron-specific enolase and has been found to contain membrane-bound neurosecretory granules within the tumor cell. In the past, the correct diagnosis often was not obtained until an extensive recurrence was noted. MCC was often misdiagnosed as BCC, lymphoma, adnexal carcinoma, or carcinoma metastatic to the skin from another primary site (i.e., small cell carcinoma of the lung or medullary carcinoma of the thyroid).12
Melanoma
Melanoma arises from melanocytes, which are present in the epidermis as well as in other parts of the body including the eye and respiratory, gastrointestinal, and genitourinary tracts.
![]() PATTERNS OF SPREAD
PATTERNS OF SPREAD
The patterns of spread for individual anatomic sites are outlined in the Selection of Treatment Modality section. Some lesions remain confined to the epidermis (CIS) and may involve a large area of skin. Large in situ lesions occur more often on the trunk; however, small areas of CIS are common on the head and neck.
Basal Cell and Squamous Cell Carcinomas
Both BCC and SCC usually are well differentiated, and most have an indolent growth with distinct margins; a small proportion are poorly differentiated and grow rapidly. BCC occurs more frequently around the central portion of the face, whereas SCC occurs more often on the ears, preauricular and temporal area, scalp, and skin of the neck.
Most lesions remain superficial and invade the adjacent epidermis in a circumferential growth pattern. Invasion of the dermis usually is confined to the superficial (papillary) dermis. Eventually, penetration of the reticular dermis, subcutaneous tissues, and other underlying structures occurs. A few skin carcinomas tend to grow beneath the skin, and the surface lesion gives little indication of their extensive growth; this is more often seen in recurrent tumors. Most early BCCs and SCCs show an orderly invasion of the superficial dermis, which allows successful local therapy. Both BCCs and SCCs invade cartilage and bone, develop PNI spread, and eventually enter the lymphatics, although the latter is not common. BCC, in particular, has a low incidence of lymphatic involvement unless it is recurrent, whereas the incidence of lymph node spread for SCC is estimated to be 10% to 15%.
SCCs may develop distant metastases, whereas BCCs rarely produce metastases.
Metatypical BCC is intermediate between BCC and SCC as far as recurrence rates and the risk of metastases.13
Spindle cell tumors of the skin have a gross appearance and growth pattern similar to SCCs.
Carcinoma of the Sweat Gland
Carcinoma of the sweat gland occurs with equal frequency in males and females. It predominantly affects elderly people, although it may occur even in early adulthood. The lesion is generally a subcutaneous nodular mass, which may be solitary or multiple, and the larger lesions may be ulcerated. Carcinoma of the sweat gland most often occurs on the eyelid, face, and scalp. The growth rate varies from indolent to rapid. The tumor may be present for several years with little change and then suddenly begin to enlarge. PNI is frequent. Regional and distant metastases may develop. Scalp lesions are the ones most likely to develop metastases to regional lymph nodes.
Recurrence after excision is frequent, and often multiple recurrences are reported.14,15 Little information exists on the response to RT.15 In our limited experience, carcinoma of the sweat gland is sufficiently radioresponsive to justify RT, particularly in association with excision.
The mucin-producing sweat gland adenocarcinoma is a rare tumor. The eyelid is the primary site in about one-half of cases and the face and scalp in another one-fourth of cases. The tumor presents most often in middle-aged men. Wright and Font16 reported 21 cases that originated on the eyelid. Eight patients (38%) developed one or more local recurrences, one patient died with extensive persistent disease in the face after a 15-year interval, and only one patient had metastasis to the submandibular lymph nodes successfully treated by radical neck dissection.16 Regional or distant metastasis is a relatively infrequent event for lesions arising in the head and neck area.
Sebaceous Gland Carcinoma
Sebaceous gland carcinoma is rare. It occurs most often on the eyelids, predominantly on the upper lid in elderly women, although the lower lid and caruncle are also sites of origin; it may occur on other parts of the head and neck skin as well. It is often indolent in its growth; however, it may be locally aggressive and develop regional and distant metastases. Local recurrence is common after excision because the lesions often have significant deep and lateral spread beyond the obvious lesion. Metastasis to regional lymph nodes is reported in about 20% of cases, and a small percentage of patients develop distant metastases.17 Inadequate treatment often occurs because of incorrect histologic diagnosis.
Keratoacanthoma
Keratoacanthoma benign lesions start as a firm, round skin nodule and grow to 1 to 2 cm in a short time, usually a few weeks. As the lesion matures, the center becomes separate and can be removed, revealing a small crater. The lesion occurs most often in the exposed area of the head and neck. Keratoacanthoma is an unlikely diagnosis for a lesion of the lip vermillion. Typically, keratoacanthoma is twice as common in men as women. It occurs most often in patients older than 40 years of age, although it may be seen as early as the second decade of life.
Basal Cell Nevus Syndrome
The basal cell nevus syndrome is an autosomal-dominant disorder with a high level of penetrance but a variable clinical picture. The clinical syndrome may be composed of any or all of the following:
• Multiple BCCs (differing only in their tendency to develop at an early age and on unexposed skin areas)
• Jaw cysts (common)
• Palmar or plantar pits
• Skeletal abnormalities (short fingers, hypertelorism)
• Ectopic calcification
• Eye muscle palsies
• Hamartomas
• Epidermal cysts
The age at onset is frequently in the second or third decade of life, and a family history is often positive for the disorder.18
Merkel Cell Carcinoma
MCC occurs primarily in white men between 60 and 80 years of age.19 The lesion often presents as a painless, raised skin nodule or mass that is red, pink, or blue and may have diffuse margins, and is covered with an intact epidermis. Most lesions are ≤2 cm in size at diagnosis.19 Human polyomavirus is thought to be etiologic in a significant proportion of patients; human polyomavirus DNA in the MCC cells may be associated with an improved prognosis.19
MCC displays an aggressive growth pattern. Mojica et al.20 reported on 1,665 patients from the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database and observed the following extent of disease at diagnosis: localized, 55%; positive regional nodes, 31%; distant metastases, 6%; and no data, 8%.
Melanoma
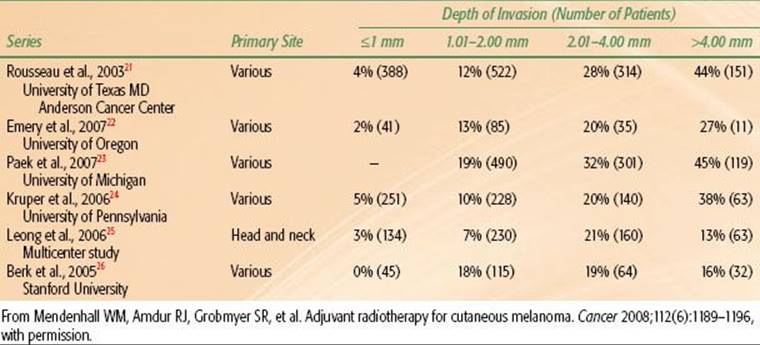
Melanoma is more aggressive and prone to metastasize to regional lymph nodes and exhibit hematogenous dissemination compared with BCC and SCC. The likelihood of regional and/or distant metastases is related to the depth of invasion of the primary tumor. The incidence of positive sentinel lymph node biopsies (SLNBs) versus depth of penetration is depicted in Table 33.1.
Lymphatics
The overall risk of lymphatic metastases is estimated to be 10% to 15% for cutaneous SCC of the skin. The risk increases with the size of the lesion, depth of the penetration, histologic grade, and recurrence.
The risk of lymph node metastases from previously untreated BCCs is <1% and is not related to size, depth of penetration, or histologic subtype; the risk increases for recurrent BCCs, especially those with multiple recurrences over several years. Lymph node metastases, however, are seen on rare occasions without a history of recurrence, and an interval of several years may occur between the treatment of the primary lesion and the appearance of involvement in lymph nodes. When they do occur, lymph node metastases often are solitary.
![]() CLINICAL PICTURE
CLINICAL PICTURE
Presenting Symptoms
The common history for BCC or SCC is a slowly enlarging growth on or just beneath the skin surface. Often a history exists of a sore that will not completely heal. Other symptoms such as bleeding or pain are unusual until the lesion becomes large, and even then symptoms are relatively mild and infrequent. Patients with PNI may complain of paresthesia, especially the sensation of worms crawling under skin (formication). PNI is usually observed with midface lesions and most often involves cranial nerves V2 and/or VII.6 Advanced, neglected lesions with bone and cartilage destruction, orbit invasion, and regional metastases may be seen; these advanced lesions often produce few, if any, symptoms, and patients simply delay consulting a physician.
Melanomas usually present as a pigmented skin lesion associated with a change in color, shape, and/or size. Although most probably arise de novo, some may arise in a previously benign nevus. Occasionally, patients present with regional or distant metastases without an apparent primary tumor.
TABLE 33.1 PRIMARY DEPTH OF INVASION VERSUS SENTINEL LYMPH NODE BIOPSY POSITIVITY
Physical Examination
The site, size, and mobility of the primary lesion is documented. Depending on location, evidence of PNI is assessed as well as any findings that might suggest involvement of underlying bone on cartilage. The regional lymph nodes must be carefully examined, even though they are not often involved. Because of the infrequent appearance of regional lymphatic metastases, and because cases of skin cancer often are not followed diligently, lymph node metastases frequently are missed. Although lymphatic metastases may appear within a few months of the management of the primary lesion, in some cases many years intervene before the regional lymph nodes become apparent. It is not at all unusual for ≥5 years to intervene between the primary lesion and the appearance of metastases. Patients with chronic lymphocytic leukemia and concomitant skin cancer often have enlarged lymph nodes from both processes and may have elements of SCC and leukemia in the same lymph nodes.
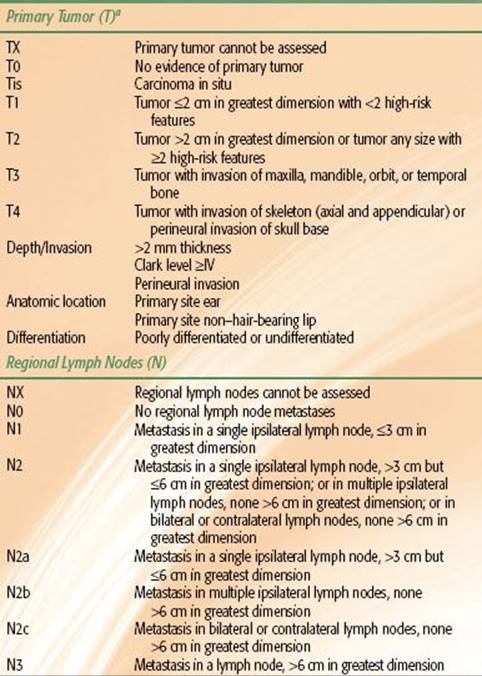
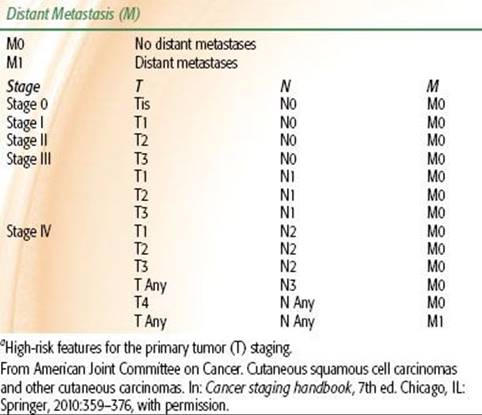
TABLE 33.2 2010 AJCC STAGING SYSTEM FOR BASAL CELL AND SQUAMOUS CELL CARCINOMAS
![]() METHODS OF DIAGNOSIS AND STAGING
METHODS OF DIAGNOSIS AND STAGING
Biopsy should be performed on the majority of lesions before deciding on treatment. We do not always insist on biopsy for elderly patients who have a typical skin carcinoma and are to be treated by RT.
Small lesions occurring on the free skin areas (i.e., not involving the eyelid, ear, or periorbital areas) usually can undergo biopsy and be treated simultaneously with surgical excision. Larger lesions, or those involving areas where functional or cosmetic deficit might occur from excision, first undergo biopsy with a small excisional biopsy or with a skin punch. Biopsy with a skin punch should include the subcutaneous fat; punch biopsy is contraindicated when differentiating between keratoacanthoma and SCC because of the small sample size.
The following is a partial list of conditions to be considered in the differential diagnosis of BCC and SCC:
• Senile keratosis
• Keratoacanthoma
• Nonpigmented nevi
• Melanoma
• Cutaneous horn
• Psoriasis
• Lymphoma (mycosis fungoides)
• Soft tissue sarcomas (dermatofibrosarcoma protuberans)
• Hemangiosarcoma
• Metastatic carcinoma
• Adnexal carcinoma of skin
• MCC
Staging
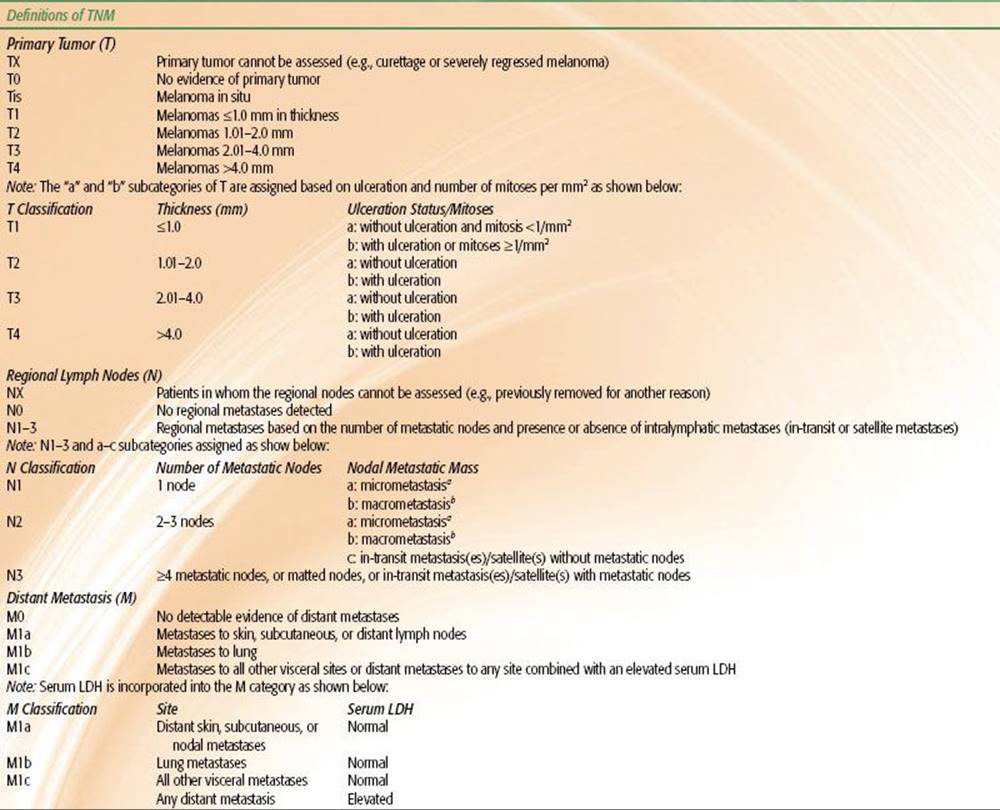
The 2010 American Joint Committee on Cancer (AJCC) systems for BCC/SCC and melanoma are depicted in Tables 33.2 and 33.3, respectively. Although there is an AJCC staging system for MCC, we prefer the Yiengpruksawan system for this rare entity because of its simplicity: stage I, localized; stage II, regional lymph node metastases; and stage III, distant metastases.27
Diagnostic Imaging
Computed tomography (CT) and magnetic resonance imaging (MRI) are only necessary in a carefully chosen group of patients being treated for skin cancer. CT is the primary modality for showing bone invasion and nodal metastases. High-resolution MRI is better than CT for demonstrating PNI.28 Positron emission tomography (PET) is useful to detect regional and distant metastases in patients with MCC and melanoma.
![]() SELECTION OF TREATMENT MODALITY
SELECTION OF TREATMENT MODALITY
Basal Cell and Squamous Cell Carcinomas
The likelihood of cure is similar after surgery or RT for early-stage BCCs and SCCs.29 Therefore, selection of one modality over another is based on other parameters such as function, cosmesis, age of the patient, convenience, cost, availability of appropriate RT equipment, and the wishes of the patient. Patients with advanced cancers are often best treated with surgery and adjuvant RT if the cancer is resectable and the functional and cosmetic outcomes are acceptable.
Radiotherapy Alone
Small skin cancers located on “free skin,” such as the cheek or forehead, may be easily excised with a good cosmetic result and minimal inconvenience; therefore, surgery is usually the treatment of choice for such lesions. It is also desirable to avoid RT in young patients because the late effects of irradiation progress gradually with time and, with very long-term follow-up, may be associated with a suboptimal cosmetic result compared with resection and reconstruction. In contrast, resection of an early-stage skin cancer of the eyelid, external ear, or nose may result in a significant cosmetic deformity and necessitate complex reconstruction that compares unfavorably with RT. This is particularly relevant in the case of older patients who have a limited life expectancy and who are at higher risk for a perioperative complication.
Patients with locally advanced skin cancers present a difficult problem because, although the likelihood of cure may be better with combined RT and surgery in some situations, the cosmetic result is sometimes unacceptable. Patients receive postoperative RT if surgery is indicated and feasible. If surgery is not feasible, the patient is managed with RT alone. Although it might seem that the risk of bone and/or cartilage necrosis would be high after RT for a skin cancer invading these structures, the observed risk of complications is relatively low.30,31 Exceptions are advanced cancers of the scalp and those overlying the anterior aspect of the tibia where there is little tissue between the skin surface and the bone. Following definitive RT, bone exposure is likely and may progress to an osteoradionecrosis (ORN) requiring surgical intervention.
Patients with lesions associated with clinical PNI with gross tumor extending to sites that render complete resection unlikely or unfeasible, such as the cavernous sinus, are treated with RT alone. Subtotal resection does not enhance the likelihood of cure and only increases the morbidity of treatment.
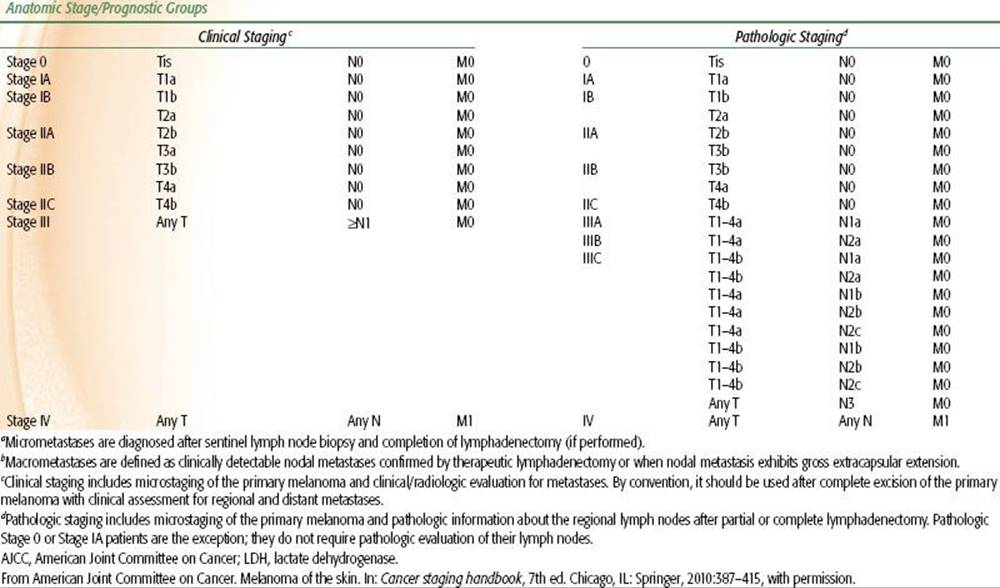
TABLE 33.3 2010 AJCC STAGING SYSTEM FOR MELANOMA
Adjuvant Radiotherapy
Postoperative RT is added after surgery if pathologic examination of the surgical specimen reveals findings indicative of a high risk for local recurrence, such as close or positive margins and/or invasion of nerve, cartilage, or bone. A significant proportion of patients with positive margins after resection of an early-stage skin cancer may never develop a local recurrence. Postoperative RT may be withheld if the lesion is a BCC, the primary site is located on the free skin, the patient is reliable and will return for close follow-up, and if salvage treatment would have a high likelihood of eradicating recurrent tumor with a good cosmetic result. BCCs of the nose, eyelid, or ear and lesions that would have immediate access to major nerve trunks are usually retreated immediately when resection margins are positive. The risk of recurrence is probably greater than for lesions of the free skin, and the consequences of recurrence may be significant. Observation is particularly attractive for the elderly patient in poor medical condition who may not live to experience a local recurrence. Metatypical BCCs behave more aggressively than BCCs, and those with positive margins after surgery should be re-excised and/or treated with postoperative RT.
Patients with BCC and focal incidental PNI involving a small nerve trunk and widely negative margins may be safely observed. Otherwise, patients with incidental PNI should be considered for postoperative RT, particularly if the lesion is SCC.
The usual policy for patients with positive margins from SCC is immediate retreatment by either re-excision, RT, or both, depending on the situation. There is evidence that this approach leads to a reduced risk of metastasis and reduced likelihood of death from cancer.
Management of Regional Lymph Node Metastases
Carcinoma of the skin metastatic to the parotid lymph nodes is managed as a high-grade parotid neoplasm, usually with superficial or total parotidectomy followed by postoperative RT.29,32–34 If only one node is involved and there is no extracapsular extension, it may be safe to withhold RT. However, if the tumor recurs in the parotid area after parotidectomy, the likelihood of salvage is remote.32 Therefore, it has been our practice to use combined treatment in all patients. If the parotid lymph node metastasis is fixed and thought to be incompletely resectable, the patient is treated with high-dose preoperative RT (6,000 to 7,000 cGy) followed by parotidectomy. RT alone is used only in patients who are inoperable because of tumor extent or poor medical condition.
Cervical lymph node metastases are managed just as they would be for other carcinomas of the head and neck. Neck dissection alone is adequate treatment if only one node is involved and there is no extracapsular extension. If two or more nodes contain tumor or if extracapsular extension is noted, neck dissection is followed by postoperative RT.
Merkel Cell Carcinoma
The preferred treatment for MCC is resection of the primary tumor and any clinically positive regional nodes. Our bias is to treat both the primary site and regional lymphatics with postoperative RT. The dose-fractionation schedule is the same as that employed for SCC. The role of adjuvant chemotherapy is ill defined. Patients who should be considered for adjuvant chemotherapy are those with positive nodes and/or satellite lesions. Drugs employed are often those used for small cell carcinomas and include etoposide and cisplatin.
Melanoma
The preferred treatment for melanoma is resection of the primary lesion and any grossly positive regional nodes. Patients with desmoplastic melanoma are usually suitable for surgery alone unless there is extensive PNI associated with a lesion near a named nerve, in which case postoperative RT is considered. Postoperative RT is also employed to treat the primary site when there are in-transit metastases and the margins are equivocal. Patients who have lesions with a depth of invasion exceeding 1 mm and clinically negative nodes should be considered for SLNB. We also perform SLNB for lesions with depth of invasion ≤1 mm if there is also ulceration or invasion into Clark’s level IV or V. Adjuvant postoperative RT is often employed for positive regional nodes. Patients who are at high risk for regional lymph node metastases and are unable to undergo SLNB and/or elective node dissection should be considered for elective nodal irradiation of the first-echelon lymphatics. The dose-fractionation schedule is usually the MD Anderson hypofractionation technique consisting of 3,000 cGy in five fractions over 2.5 weeks with a reduction off of the spinal cord and/or central nervous system (CNS) at 2,400 cGy.35 Alternatively, conventional fractionation may be employed if late effects and suboptimal cosmesis are a concern.36
Definitive RT using orthovoltage RT or electrons may be employed for elderly patients with lentigo maligna or lentigo malignant melanoma where excision is not feasible because of cosmesis function and/or medial comorbidities.37–39 The dose-fractionation schedules are the same as those described for postoperative RT.
![]() TREATMENT TECHNIQUES
TREATMENT TECHNIQUES
Treatment techniques vary for primary lesions and for regional lymph node metastases.
Primary Lesion
RT of the primary lesion is usually accomplished using one of three basic external-beam techniques (orthovoltage RT, electron beam, and high-energy x-rays or photons) or with an interstitial implant either alone or in combination with external-beam techniques. Most early skin cancers are managed with orthovoltage RT with beam energies of 100 to 250 Kvp. The advantages of this technique, compared with electron beam, are that the maximum dose is at the skin surface; bolus is not required; there is less beam constriction both at the surface and at depth so that smaller fields can be used (Fig. 33.1); shielding of the eye is easier and more effectively accomplished, particularly if electron energies ≥10 MeV are used (Table 33.4)40; it is less expensive; and the likelihood of tumor control may be higher, possibly as a result of increased radiobiologic effectiveness (RBE) but more likely because of technical problems that are difficult to overcome when using electrons alone. The disadvantages of orthovoltage x-rays, compared with electron beam, are that there is a higher dose to deeper tissues and to underlying bone and cartilage. The latter problem can be largely eliminated by using heavily filtered orthovoltage beams for tumors involving or overlying cartilage or bone. Another significant problem associated with orthovoltage RT is that most radiation oncology departments do not have an orthovoltage machine, and thus it is unavailable. Electron beam is usually used for lesions of the scalp to reduce the dose to the underlying brain; orthovoltage x-rays are used to treat most other skin cancers. Orthovoltage RT is particularly useful for lesions of the head and neck.
FIGURE 33.1. A: 250-kVp x-rays (HVL 1.4 mm Cu) with secondary collimation on the phantom surface. Source-to-surface distance (SSD) = 50 cm; isodose %: 95, 90, 80, 70. B: 6-MeV electron beam with secondary collimation 5 cm above the phantom surface (at the level of the electron cone). Source of collimator distance (SCD) = 95 cm; SSD = 100 cm; isodose %: 95, 90, 80, 70, 50. C: 6-MeV electron beam with tertiary collimation on the phantom surface. SSD = 100 cm; SCD = 100 cm; isodose %: 95, 90, 80, 70, 50.
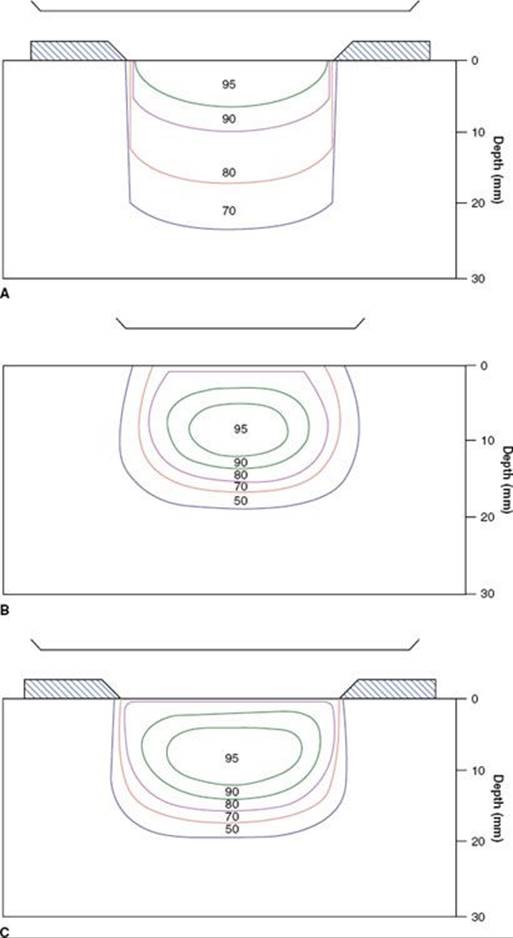
Before treatment with either electron-beam or orthovoltage x-rays, a customized lead mask is constructed to collimate the beam directly on the skin surface. A 1.0- to 1.5-cm margin is adequate for a well-defined T1 lesion treated with orthovoltage RT. An additional 1 cm is added to the margin when the electron beam is used to account for beam constriction. For larger and/or ill-defined tumors, a 2-cm margin is usually necessary. If the tumor is located near the eye, a gold-plated lead eye shield is placed directly on the anesthetized cornea to minimize the irradiation dose to underlying structures (Fig. 33.2). The dose beneath the eye shield is depicted for orthovoltage x-rays and electron beams of various energies in Table 33.4. Isodose distributions for the two techniques are shown in Figure 33.1; note that the dose distribution is better for orthovoltage RT than for electron beam, particularly if a lead mask is not used with the electron beam.
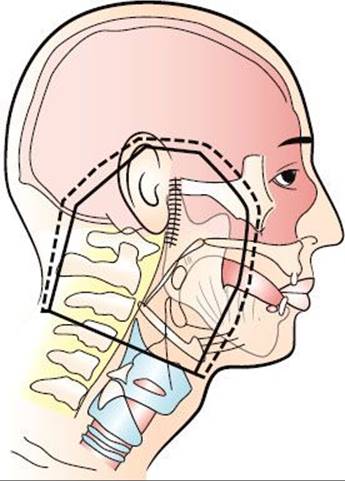
Advanced skin cancers that are deeply invasive are often treated with high-energy photons to adequately cover the deep extent of the tumor. Bolus is used to ensure an adequate surface dose. Field arrangement varies with primary site. For example, a wedge-pair technique may be used for lesions involving the external ear, whereas a three-field technique (similar to that used for paranasal sinus cancer) is frequently used for skin cancers with extension proximally along the second division of the fifth cranial nerve. The target volume includes the entire course of the involved nerve, which would include the gasserian ganglion in this case, to the brain stem. Intensity-modulated radiotherapy (IMRT) may be used in some patients to produce a more conformal dose distribution to reduce the dose to surrounding normal structures. Proton-beam radiotherapy may also be used to produce a very conformal dose distribution with steep dose gradients to limit the dose to the visual apparatus and the CNS.41,42
The extent of RT fields for patients with PNI varies with histology and the extent of PNI. Patients with BCC or SCC and focal incidental PNI are treated with a wide local field. Those with extensive incidental PNI are treated with fields that include the at-risk nerves to the skull base, particularly if margins are positive or equivocal. The at-risk nerves receive 50 to 70 Gy depending on the extent of PNI and margin status. Patients with clinical PNI receive definitive RT to fields that include the primary site and involved nerves to the skull base.
The regional lymph nodes are electively irradiated if the suspected risk of subclinical disease exceeds 10% to 15%.43 Patients with SCC and PNI should be considered for elective nodal RT, particularly if the lesion is located on the head or neck.
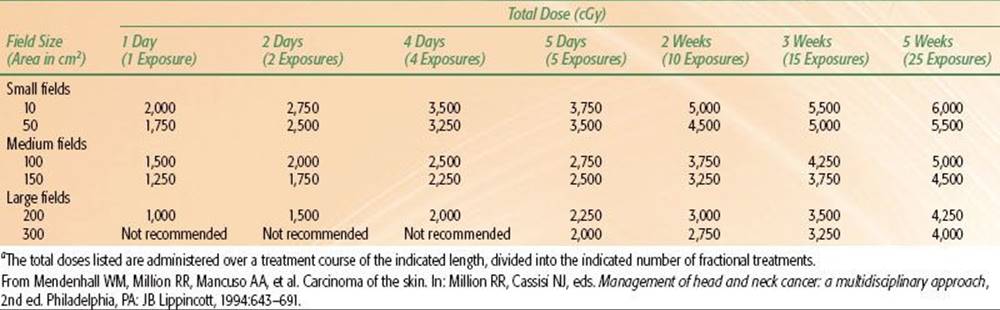
Dose-fractionation schedules used for irradiation of skin cancers are outlined in Table 33.5. We have recently used twice-daily RT with megavoltage photons or protons at 1.2 Gy per fraction to doses of 74.4 Gy in a continuous course with a minimum 6-hour interfraction interval for advanced lesions near radiosensitive structures. The suggested maximum doses for short-course orthovoltage RT are listed in Table 33.6.
TABLE 33.4 OCULAR PROTECTION: DOSE BENEATH THE EYE SHIELD
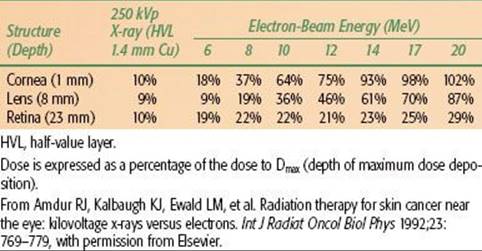
TABLE 33.5 GUIDELINES FOR SELECTION OF EXTERNAL-BEAM DOSE
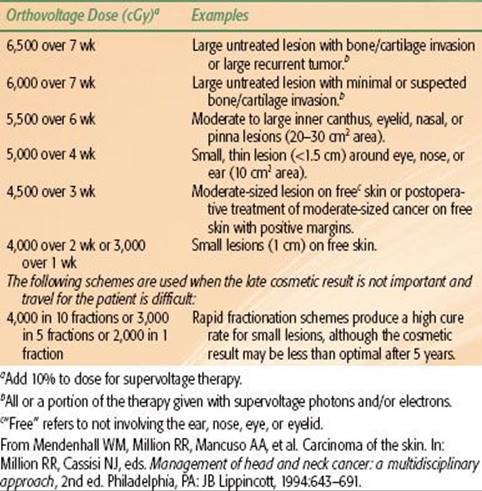
FIGURE 33.2. A: 1-cm é 1-cm basal cell carcinoma of the midportion of the lower lid. B: Treatment setup that was used to irradiate the patient. Arrows indicate the field edge. ES, eye shield.
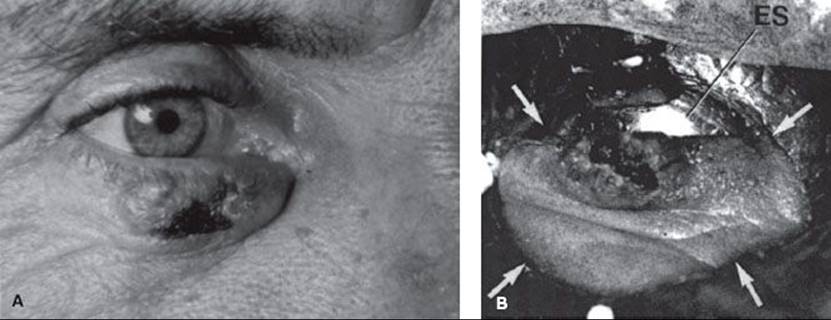
TABLE 33.6 SUGGESTED MAXIMUM SKIN DOSESA FOR PALLIATION WITH 250 KVP X-RAYS (BELOW MOIST DESQUAMATION LEVEL FOR THE AVERAGE PATIENT)
Parotid-Area Lymph Node Metastases
The parotid gland and upper neck are treated with an en face mixed beam of 6-MV x-rays and high-energy electrons (usually 20 MeV), weighted 1.0:0.67 in favor of the electron beam. The electron-beam field is 1 cm larger than the x-ray field to account for the beam constriction of the electrons, except where the field abuts the low-neck field (Fig. 33.3). This field arrangement is treated to 4,600 cGy in 23 fractions, specified at a depth of 4 to 5 cm from the skin surface. An anterior field is matched to the lateral mixed-beam field to treat the ipsilateral low neck and is irradiated to 5,000 cGy in 25 fractions, specified at Dmax.
At 4,600 cGy tumor dose, the primary field is reduced off of the spinal cord, and the dose to the tumor bed is boosted with an anteriorly angled 20-MeV electron-beam field or an appositional mixed-beam field with an abutting posterior electron strip. The final dose for patients treated at 200 cGy per fraction with negative margins is 6,000 cGy; with positive margins, 6,600 cGy; and with gross residual disease, 7,000 cGy. If the dose per fraction is reduced to 180 cGy, the total dose is increased by 500 cGy. An alternative to the mixed electron–photon beam is a wedge-pair technique using 4 to 6 MV photon beams. IMRT may also be considered to reduce the dose to the temporal lobe and cerebellum, particularly if the margins are positive and/or tumor involves the deep lobe of the parotid.
Axillary Metastases
Axillary lymph node metastases are irradiated with opposed anterior and posterior portals that include the supraclavicular fossa. The dose-fractionation schedule is 4,500 cGy in 25 fractions with a reduction and boost depending on margin status.
Ilioinguinal Lymph Node Metastases
Ilioinguinal lymph nodes are usually irradiated with IMRT to reduce the volume of tissue irradiated, particularly small bowel. The femoral and external iliac vessels are contoured on the treatment planning CT and a 1.5- to 2-cm margin is added to obtain the clinical target volume (CTV).
![]() FOLLOW-UP AND MANAGEMENT OF RECURRENCE
FOLLOW-UP AND MANAGEMENT OF RECURRENCE
After RT, patients are evaluated every 2 to 3 months for the first and second years, every 4 months for the third year, every 6 months for the fourth and fifth years, and annually thereafter. Chest roentgenograms are obtained annually, and a follow-up CT, MRI, and/or PET is obtained as needed for patients irradiated for advanced lesions.
Treatment of local-regional recurrences after RT alone or combined with surgery is usually resection. If a local recurrence is amenable to en bloc surgical resection with frozen section control of the margins, this may be preferable for selected cases. Local recurrences after RT may be significantly more extensive than is clinically apparent; thus, wide margins are preferred. Retreatment with RT is rarely indicated because of the high likelihood of complications. The most common scenario for recurrence in the neck nodes is failure in a solitary submandibular location; the success rate of salvage neck dissection is high in this circumstance.
FIGURE 33.3. Typical en face mixed-beam field used in the treatment of parotid node metastases to encompass the entire parotid bed and surgical scar. Patients are treated supine with a face mask made of low-temperature thermal plastic (polycaprolactone) for immobilization and a lead lollipop to decrease the dose to the contralateral salivary gland. A bolus of petrolatum-coated gauze is applied to all scars.
![]() RESULTS
RESULTS
Basal Cell and Squamous Cell Carcinomas
Most series reporting the results of treatment for skin cancer contain a preponderance of early lesions and do not employ a staging system, making it difficult to compare various series and the relative efficacy of different treatment modalities.
Primary Lesion
The likelihood of local control after irradiation for skin carcinomas of various sizes managed at the Mallinckrodt Institute of Radiology is outlined in Table 33.7.44 The probability of local control is higher for smaller lesions, for BCC compared with SCC, and for previously untreated lesions compared with recurrent cancers. The results of RT using various beam energies are listed in Table 33.8; treatment with orthovoltage irradiation yields local control rates that are as good as, or better than, local control rates yielded by other treatment modalities.44
Schulte et al.45 reported on 1,113 patients treated with orthovoltage RT for 1,267 skin cancers and followed for a median of 82 months (Table 33.9). Patients were usually treated at 5 Gy per fraction. The incidence of soft tissue necrosis was 6.3%; 83% healed with conservative treatment.45
Al-Othman et al.46 reported on 85 patients with 88 clinical T4 SCCs (37), BCCs (41), and metatypical BCCs (10) treated with definitive RT at the University of Florida (Gainesville, FL) between 1964 and 1997. Forty-three lesions were previously untreated, and 45 cancers were recurrent after prior surgery. The 5-year outcomes were as follows: local control, 53%; ultimate local control, 90%; regional control, 93%; ultimate regional control, 100%; distant metastasis-free survival, 95%; cause-specific survival, 76%; and overall survival, 56%. Thirteen (15%) of 85 patients developed a severe treatment-related complication.
Balamucki et al.47 reported on 216 patients treated at the University of Florida for skin carcinomas with incidental (107 patients) or clinical (109 patients) PNI; median follow-up for living patients was 6 years (range, 0.6 to 2.3 years). One hundred eighty-five patients (86%) had SCCs, and the remainder had BCCs or metatypical BCCs. Patients with incidental PNI were treated with surgery and postoperative RT (99 patients), preoperative RT and surgery (4 patients), and RT alone (4 patients). Eight patients received adjuvant chemotherapy. Twenty six of 107 patients (24%) presented with clinically positive nodes. The outcomes for patients treated with incidental PNI are depicted in Table 33.10. Seventeen of 107 patients (16%) developed treatment complications. Patients with clinical PNI were treated with surgery and postoperative RT (58 patients), preoperative RT and surgery (2 patients), or RT alone (49 patients). Fifteen of 109 patients (14%) presented with clinically positive nodes. The outcomes for patients treated for clinical PNI are depicted in Table 33.11. Fourteen of 62 patients (23%) who had achieved continuous local control experienced symptomatic improvement in clinical neuropathic symptoms after treatment. Thirty nine of 109 patients (36%) developed treatment complications.
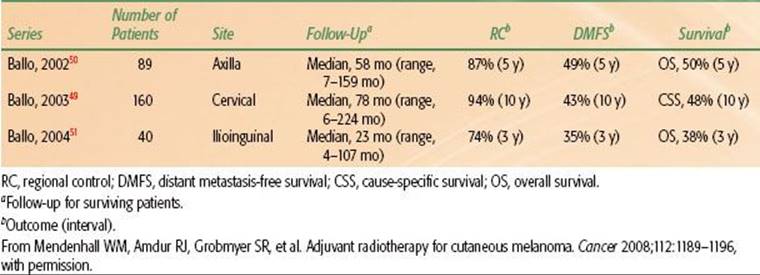
Galloway et al.48 reported on a subset of these patients and compared their outcomes with radiographic extent of PNI and found that the extent of PNI was inversely related to the likelihood of local control and survival (Table 33.12).
TABLE 33.7 LOCAL TUMOR CONTROL WITH RADIOTHERAPY ACCORDING TO SIZE, CELL TYPE, AND PRESENTATION (FROM MALLINCKRODT INSTITUTE OF RADIOLOGY—339 PATIENTS)
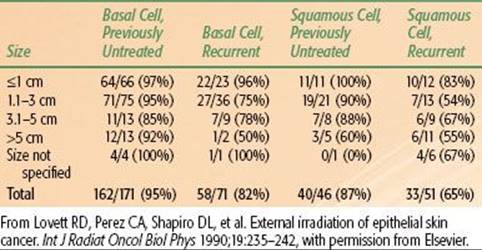
TABLE 33.8 LOCAL CONTROL RATES ACCORDING TO EXTERNAL-BEAM TECHNIQUE (FROM MALLINCKRODT INSTITUTE OF RADIOLOGY—339 PATIENTS)
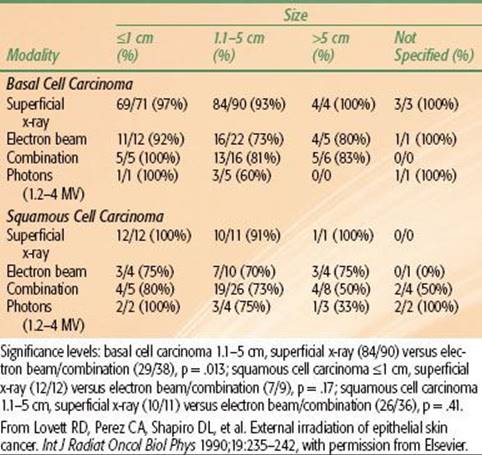
TABLE 33.9 RAW AND CUMULATIVE RECURRENCE RATES OF BASAL CELL AND SQUAMOUS CELL CARCINOMAS AFTER SOFT X-RAY THERAPY
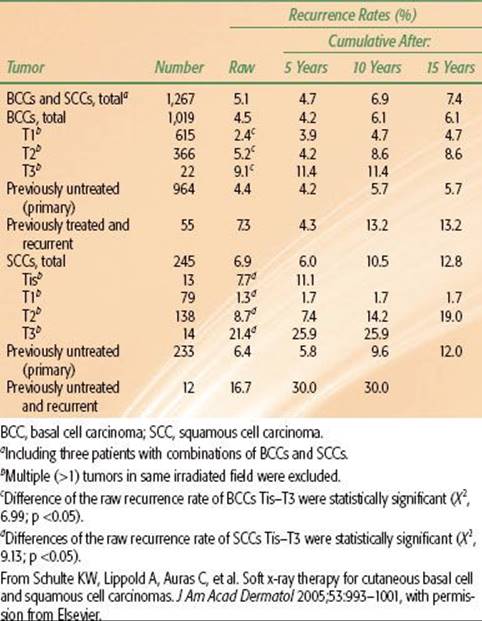
TABLE 33.10 OUTCOMES AFTER TREATMENT FOR SKIN CARCINOMA WITH INCIDENTAL PERINEURAL INVASION (107 PATIENTS)
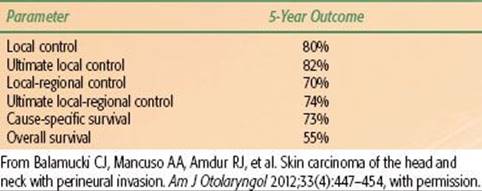
TABLE 33.11 OUTCOMES AFTER TREATMENT FOR SKIN CARCINOMA WITH CLINICAL PERINEURAL INVASION (109 PATIENTS)
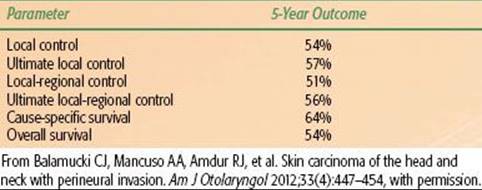
TABLE 33.12 CLINICAL PERINEURAL INVASION: 5-YEAR OUTCOME VERSUS PRETREATMENT RADIOGRAPHIC FINDINGS (45 PATIENTS)
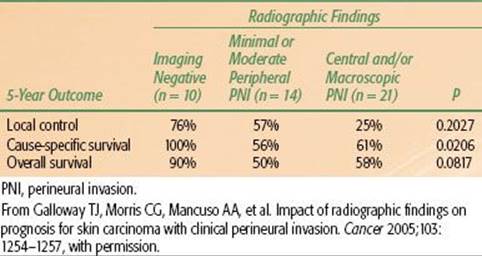
TABLE 33.13 MERKEL CELL CARCINOMA 5-YEAR OUTCOMES VERSUS STAGE
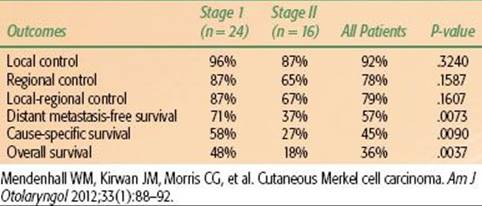
TABLE 33.14 OUTCOMES AFTER SURGERY AND POSTOPERATIVE RADIOTHERAPY AT THE UNIVERSITY OF TEXAS MD ANDERSON CANCER CENTER FOR LYMPH NODE–POSITIVE MELANOMA PATIENTS
Regional Nodes
Veness et al.32 reported on 167 patients treated at Westmead Hospital (Sydney, Australia) between 1980 and 2000 for cutaneous SCCs metastatic to the parotid and/or cervical nodes. Twenty-one patients (13%) were treated with surgery alone, and the remainder received surgery and adjuvant RT. The median time to recurrence after treatment was 8 months. The 5-year local-regional recurrence and disease-free survival rates were as follows: surgery and RT, 20% and 73%, and surgery alone, 43% and 54%, respectively. Multivariate analysis revealed that multiple positive nodes and treatment with surgery alone were significantly associated with decreased survival.
Hinerman et al.33 reported on 117 patients with 121 clinically positive parotids treated at the University of Florida between 1969 and 2005. Patients were treated with preoperative RT and surgery (17 parotids), surgery and postoperative RT (87 parotids), and RT alone (17 parotids). The 5-year outcomes were as follows: local (parotid) control, 78%; local-regional control, 74%; distant metastasis-free survival, 92%; disease-free survival, 70%; and overall survival, 54%. The 5-year local-regional control rate was 83% after surgery and postoperative RT versus 47% after preoperative RT and surgery or RT alone. Three (3%) patients developed severe complications.
Merkel Cell Carcinoma
Mendenhall et al.19 reported on 40 patients treated with RT alone (3 patients) or combined with surgery (37 patients) at the University of Florida between 1984 and 2009. Eleven patients received adjuvant chemotherapy. Twenty-four patients had stage I disease, and 16 patients had stage II MCC. Median follow-up on survivors was 4.2 years (range, 2.2 to 14.2 years). No patient was lost to follow-up. The 5-year outcomes are depicted in Table 33.13. No severe late complications were observed.
Melanoma
One of the largest experiences with adjuvant RT for melanoma has been reported by investigators from the MD Anderson Cancer Center (Houston, TX; Table 33.14). Although RT may be used to treat gross disease, this would only occur by default and in mostly palliative situations.
Tsang et al.37 reported on 36 patients treated between 1968 and 1988 with definitive RT for lentigo maligna at the Princess Margaret Hospital (Toronto, Canada) and followed for a median 6 years. The 5-year local control rate was 86%. Farshad et al.39 reported an 150 patients treated at the University of Zurich with definitive RT for lentigo maligna (93 patients), lentigo maligna melanoma (54 patients), or both (3 patients). One hundred one patients were followed for at least 2 years (mean, 8 years); the local control rate was 93%.
![]() CONCLUSIONS
CONCLUSIONS
RT is an effective modality for the primary treatment of skin cancer as well as in the adjuvant setting. It is used to treat the primary skin lesion when resection would result in an unacceptable functional and/or cosmetic outcome as well as in the patient who is medically unsuitable for, or who declines, surgery. Adjuvant RT is usually indicated after surgery for close or positive margins, PNI, bone and/or cartilage involvement, metastatic parotid-area lymph nodes, multiple lymph node metastases, and extracapsular extension.
![]() REFERENCES
REFERENCES
1. American Cancer Society. Skin cancer: basal and squamous cell overview. Available at: http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/OverviewGuide/index. Accessed March 29, 2011.
2. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300.
3. Yoffey JM, Courtice FC. Lymphatics, lymph and lymphoid tissue. Cambridge, MA: Edward Arnold, 1956.
4. Taylor BW Jr, Brant TA, Mendenhall NP, et al. Carcinoma of the skin metastatic to parotid area lymph nodes. Head Neck 1991;13:427–433.
5. Toker C. Trabecular carcinoma of the skin. Arch Dermatol 1972;105:107–110.
6. Mendenhall WM, Amdur RJ, Hinerman RW, et al. Skin cancer of the head and neck with perineural invasion. Am J Clin Oncol 2007;30:93–96.
7. Evans HL, Smith JL. Spindle cell squamous carcinomas and sarcoma-like tumors of the skin: a comparative study of 38 cases. Cancer 1980;45:2687–2697.
8. Ackerman AB. Histopathology of keratoacanthoma. In: Andrade R, Gumport SL, Popkin GL, et al., eds. Cancer of the skin: biology, diagnosis, management. Philadelphia, PA: WB Saunders, 1976:781–796.
9. Chow WC, Cockerell CJ, Geronemus RG. Microcystic adnexal carcinoma of the scalp. J Dermatol Surg Oncol 1989;15:768–771.
10. Mayer MH, Winton GB, Smith AC, et al. Microcystic adnexal carcinoma (sclerosing sweat duct carcinoma). Plast Reconstr Surg 1989;84:970–975.
11. Requena L, Marquina A, Alegre V, et al. Sclerosing-sweat-duct (microcystic adnexal) carcinoma—a tumor from a single eccrine origin. Clin Exp Dermatol 1990;15:222–224.
12. Goepfert H, Remmler D, Silva E, et al. Merkel cell carcinoma (endocrine carcinoma of the skin) of the head and neck. Arch Otolaryngol 1984;110:707–712.
13. Schuller DE, Berg JW, Sherman G, et al. Cutaneous basosquamous carcinoma of the head and neck: a comparative analysis. Otolaryngol Head Neck Surg 1979;87:420–427.
14. Fierstein JT, Thawley SE, Druck NS, et al. Metastatic sweat gland carcinoma. Laryngoscope 1978;88:1691–1696.
15. Harari PM, Shimm DS, Bangert JL, et al. The role of radiotherapy in the treatment of malignant sweat gland neoplasms. Cancer 1990;65:1737–1740.
16. Wright JD, Font RL. Mucinous sweat gland adenocarcinoma of eyelid: a clinicopathologic study of 21 cases with histochemical and electron microscopic observations. Cancer 1979;44:1757–1768.
17. Mellette JR, Amonette RA, Gardner JH, et al. Carcinoma of sebaceous glands on the head and neck. A report of four cases. J Dermatol Surg Oncol 1981;7:404–407.
18. Southwick GJ, Schwartz RA. The basal cell nevus syndrome: disasters occurring among a series of 36 patients. Cancer 1979;44:2294–2305.
19. Mendenhall WM, Kirwan JM, Morris CG, et al. Cutaneous Merkel cell carcinoma. Am J Otolaryngol 2012;33(1):88–92.
20. Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol 2007;25:1043–1047.
21. Rousseau DL Jr, Ross MI, Johnson MM, et al. Revised American Joint Committee on Cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol2003;10:569–574.
22. Emery RE, Stevens JS, Nance RW, et al. Sentinel node staging of primary melanoma by the “10% rule”: pathology and clinical outcomes. Am J Surg 2007;193:618–622.
23. Paek SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer 2007;109:100–108.
24. Kruper LL, Spitz FR, Czerniecki BJ, et al. Predicting sentinel node status in AJCC stage I/II primary cutaneous melanoma. Cancer 2006;107:2436–2445.
25. Leong SP, Accortt NA, Essner R, et al. Impact of sentinel node status and other risk factors on the clinical outcome of head and neck melanoma patients. Arch Otolaryngol Head Neck Surg 2006;132:370–373.
26. Berk DR, Johnson DL, Uzieblo A, et al. Sentinel lymph node biopsy for cutaneous melanoma: the Stanford experience, 1997–2004. Arch Dermatol 2005;141:1016–1022.
27. Yiengpruksawan A, Coit DG, Thaler HT, et al. Merkel cell carcinoma. Prognosis and management. Arch Surg 1991;126:1514–1519.
28. Mancuso AA, Hanafee WN. Head and neck radiology, 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2011.
29. Mendenhall WM, Million RR, Mancuso AA, et al. Carcinoma of the skin. In: Million RR, Cassisi NJ, eds. Management of head and neck cancer: a multidisciplinary approach, 2nd ed. Philadelphia, PA: JB Lippincott, 1994:643–691.
30. Million RR. The myth regarding bone or cartilage involvement by cancer and the likelihood of cure by radiotherapy. Head Neck 1989;11:30–40.
31. Mendenhall WM, Amdur RJ, Hinerman RW, et al. Radiotherapy for cutaneous squamous and basal cell carcinomas of the head and neck. Laryngoscope 2009;119:1994–1999.
32. Veness MJ, Morgan GJ, Palme CE, et al. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope 2005;115:870–875.
33. Hinerman RW, Amdur RJ, Morris CG, et al. Cutaneous squamous cell carcinoma metastatic to parotid-area lymph nodes. Laryngoscope 2008;118:1989–1996.
34. Andruchow JL, Veness MJ, Morgan GJ, et al. Implications for clinical staging of metastatic cutaneous squamous carcinoma of the head and neck based on a multicenter study of treatment outcomes. Cancer 2006;106:1078–1083.
35. Mendenhall WM, Amdur RJ, Grobmyer SR, et al. Adjuvant radiotherapy for cutaneous melanoma. Cancer 2008;112:1189–1196.
36. Chang DT, Amdur RJ, Morris CG, et al. Adjuvant radiotherapy for cutaneous melanoma: comparing hypofractionation to conventional fractionation. Int J Radiat Oncol Biol Phys 2006;66:1051–1055.
37. Tsang RW, Liu FF, Wells W, et al. Lentigo maligna of the head and neck. Results of treatment by radiotherapy. Arch Dermatol 1994;130:1008–1012.
38. Khan N, Khan MK, Almasan A, et al. The evolving role of radiation therapy in the management of malignant melanoma. Int J Radiat Oncol Biol Phys 2011;80:645–654.
39. Farshad A, Burg G, Panizzon R, et al. A retrospective study of 150 patients with lentigo maligna and lentigo maligna melanoma and the efficacy of radiotherapy using Grenz or soft X-rays. Br J Dermatol2002;146:1042–1046.
40. Amdur RJ, Kalbaugh KJ, Ewald LM, et al. Radiation therapy for skin cancer near the eye: kilovoltage x-rays versus electrons. Int J Radiat Oncol Biol Phys 1992;23:769–779.
41. Bhandare N, Monroe AT, Morris CG, et al. Does altered fractionation influence the risk of radiation-induced optic neuropathy? Int J Radiat Oncol Biol Phys 2005;62:1070–1077.
42. Monroe AT, Bhandare N, Morris CG, et al. Preventing radiation retinopathy with hyperfractionation. Int J Radiat Oncol Biol Phys 2005;61:856–864.
43. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck 2003;25:1027–1033.
44. Lovett RD, Perez CA, Shapiro DL, et al. External irradiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys 1990;19:235–242.
45. Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol 2005;53:993–1001.
46. Al-Othman MOF, Mendenhall WM, Amdur RJ. Radiotherapy alone for clinical T4 skin carcinoma of the head and neck with surgery reserved for salvage. Am J Otolaryngol 2001;22:387–390.
47. Balamucki CJ, Mancuso AA, Amdur RJ, et al. Skin carcinoma of the head and neck with perineural invasion. Amer J Otolaryngol 2012;33(4):447–454.
48. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer 2005; 103:1254–1257.
49. Ballo MT, Bonnen MD, Garden AS, et al. Adjuvant irradiation for cervical lymph node metastases from melanoma. Cancer 2003;97:1789–1796.
50. Ballo MT, Strom EA, Zagars GK, et al. Adjuvant irradiation for axillary metastases from malignant melanoma. Int J Radiat Oncol Biol Phys 2002;52:964–972.
51. Ballo MT, Zagars GK, Gershenwald JE, et al. A critical assessment of adjuvant radiotherapy for inguinal lymph node metastases from melanoma. Ann Surg Oncol 2004;11:1079–1084.