Abha Majumdar1 and Nisha Sharma Mangal1
(1)
Centre of IVF and Human Reproduction, Department of Obstetrics and Gynecology, Sir Gangaram Hospital, Rajendra Nagar, New Delhi, Delhi, 110060, India
Abha Majumdar (Corresponding author)
Email: abhamajumdar@hotmail.com
Nisha Sharma Mangal
Email: drnishamangal@gmail.com
Abstract
Prolactin is a polypeptide hormone that is synthesized in the anterior pituitary gland and secreted in a pulsatile manner. It plays central role in a variety of reproductive functions and lactation. Prolactin release in humans depends on physiological state and varies in response to different stimuli.
Hyperprolactinaemia is a common endocrinological disorder; it could be physiological, pathological or idiopathic in origin. The predominant physiological consequence of hyperprolactinaemia is suppression of pulsatile GnRH. The clinical manifestations of conditions vary significantly depending on the age and the sex of the patient. In women, it frequently leads to gonadal dysfunction including ovulatory disorder, menstrual disturbances, galactorrhoea and infertility.
Problem in diagnosing and treating hyperprolactinaemia is the occurrence of the macro-prolactin molecule, which is, although biologically inactive, yet detected as hyperprolactinaemia in most immune assays. The management of anovulatory infertility due to hyperprolactinaemia requires establishing high prolactin levels as the cause of anovulation. Dopamine agonist is the mainstay of treatment. Gonadotropins can be used as substitution therapy to induce ovulation. Resistant cases of pituitary macro-adenoma may require surgical or radiological management.
Keywords
ProlactinHyperprolactinemiaGalactorrheaInfertility
29.1 Introduction
Prolactin is an anterior pituitary hormone, which has its principal physiological action in initiation and maintenance of lactation. Hyperprolactinaemia is a condition of elevated prolactin levels in blood that could be physiological, pathological, or idiopathic in origin. Elevated prolactin levels could be associated with severe clinical manifestations on one side or be completely asymptomatic on the other side of the spectrum. It plays a central role in a variety of reproductive functions. In female reproduction, pathological hyperprolactinaemia most commonly presents as an ovulatory disorder and is often associated with secondary oligomenorrhoea or amenorrhoea.
29.2 Prevalence
It is a common endocrine disorder of the hypothalamic-pituitary axis. It occurs more commonly in women [1]. The prevalence of hyperprolactinaemia ranges from less than 1 % in an unselected adult population to as high as 9–17 % in women with reproductive diseases [2].
29.3 Prolactin Molecule
Prolactin is a 23 kDa polypeptide hormone (199 amino acids) synthesized in the lactotroph cells of the anterior pituitary gland. Its secretion is pulsatile and increases with sleep, stress, food ingestion, pregnancy, chest wall stimulation and trauma. The primary source of prolactin is anterior pituitary. Other sites include the endometrium in luteal phase and the decidua [3, 4].
29.3.1 Macro-Prolactin
Monomeric 23 kDa form is the predominant form of prolactin molecule also known as ‘little prolactin’, but it is also present in other molecular forms which differ in their bioactivity (Table 29.1). These big variants of prolactin molecule are of 50 and 150 kDa and are also known as ‘big prolactin’ and the ‘big-big prolactin’, which have high immunogenic properties but poor or no biological effect. These ‘big prolactin’ or macro-prolactin represents dimers, trimers, polymers of prolactin or prolactin-immunoglobulin immune complexes. When these big variants circulate in large amounts, the condition is referred to as ‘macro-prolactinaemia’, identified as hyper-prolactinaemia by the commonly used immunoassays. These forms rarely show any biological activity but unfortunately are detected as hyperprolactineamia in most prolactin assays [2]. In these situations, even with high levels of circulating prolactin hormone, the individual may remain clinically asymptomatic [5, 6]. Commonly used commercial assays do not detect macro-prolactin separately. Polyethylene glycol precipitation is an inexpensive way to detect the presence of macro-prolactin in the serum [1].
Table 29.1
Major forms of the prolactin molecule (the little, big, and big-big)
|
Little prolactin |
Macroprolactin |
|
Secretion is pulsatile |
Dimers, trimers or polymers/ or structural modification |
|
Biologically active form |
Poor or no biological action |
|
Monomer, molecular wt. 23 kDa |
Molecular wt. 50–150 kDa |
|
Highly immunogenic |
29.4 Biological Action
Prolactin plays a central role in variety of reproductive functions. The main biological action of prolactin is in mammary development and in inducing as well as maintaining lactation [7]. In addition, it also stimulates immune responsiveness and exerts metabolic effects [8]. It binds to specific receptors in the gonads, lymphoid cells and liver [9]. Plenty of mediators of central and peripheral origin take part in regulating prolactin secretion through a direct or indirect effect on lactotroph cells [5].
Prolactin secretion is under dual regulation by hypothalamic hormones, but the predominant signal is tonic inhibitory control of hypothalamic dopamine, which acts upon pituitary lactotroph D2 receptors. Factors affecting prolactin secretion are listed in Table 29.2 [10].
Table 29.2
Factors affecting prolactin secretion
|
Prolactin inhibiting factors |
Prolactin stimulatory factors |
|
Dopamine |
Hypothalamic peptides |
|
Gama amino butyric acid (GABA) |
Dopamine receptor antagonist |
|
Somatostatins |
Thyrotropin releasing hormone |
|
Acetylcholine |
Vasoactive intestinal peptide (VIP) |
|
Norepinephrine |
Epidermal growth factor (EGF) |
|
Histamine |
|
|
Serotonin |
29.4.1 Effect of Hyperprolactinaemia on Ovulatory Function
In females, elevated prolactin levels cause ovulatory disturbances and menstrual irregularities. The main cause of anovulation is impaired gonadotropin pulsatility and derangement of the oestrogen-positive feedback effect on LH secretion. But ovarian response to gonadotropin is well maintained in these patients [11]. It also has direct action on ovaries in regulating ovarian steroidogenesis [12]. The action of prolactin on ovaries varies in different phases of a menstrual cycle:
1.
2.
29.5 Aetiology
Hyperprolactinaemia can be physiological, pharmacological, pathological or idiopathic in origin. Physiological hyperprolactinaemia is usually mild or moderate and may cause temporary episodes of hyperprolactinaemia that do not warrant any treatment because repeat assays generally show normal prolactin levels. There are a number of pharmacological agents that may lead to hyperprolactinaemia, and discontinuation of the drug readily restores prolactin level to normal. During normal pregnancy, serum prolactin rises progressively to around 10–20 fold. Prolactinomas (prolactin-secreting adenomas) are the most frequent cause of chronic pathological hyperprolactinaemia and account for 25–30 % of functioning pituitary tumours [14]. Prolactinomas are of two types depending on their size: micro-adenomas (smaller than 10 mm) and macro-adenomas (10 mm or larger). Smaller tumours are generally very slow growing or static, but the larger ones require to be followed up regularly, and if their growth causes compression of surrounding neuronal tissue, surgical intervention may be required. A number of chronic systemic diseases also cause moderate rise in prolactin levels leading to disturbed reproductive function or galactorrhoea. Apart from these known reasons for high circulating prolactin levels, a large proportion of women presenting with symptomatic hyper-prolactinaemia are idiopathic in origin (Table 29.3) [15].
Table 29.3
Causes of hyperprolactinemia
|
I. Physiologic conditions |
|
Sleep |
|
Food ingestion |
|
Stress |
|
Pregnancy and lactation |
|
Chest wall stimulation |
|
II. Idiopathic hyperprolactinemia (30–40 % of cases) |
|
III. Hypothalamic-pituitary stalk damage |
|
Tumors: Craniopharyngioma, Meningioma, Dysgerminoma, Pineal gland tumors |
|
Empty sella syndrome |
|
Lymphocytic hypophysitis |
|
Pituitary stalk section |
|
Suprasellar surgery |
|
Irradiation |
|
Trauma |
|
IV. Pituitary hypersecretion |
|
Prolactinoma (Microadenoma and Macroadenoma) |
|
Metastatic tumors |
|
Infections such as tuberculosis |
|
Cushing disease |
|
Addison’s disease |
|
Sarcoidosis |
|
Histiocytosis |
|
Acromegaly |
|
V. Systemic diseases |
|
Chronic renal failure |
|
Hypothyroidism (primary and secondary) |
|
Ectopic production (Hypernephroma, Bronchogenic sarcoma) |
|
Epileptic seizures |
|
Cirrhosis |
|
VI. Drug-induced hyper-secretion |
|
1. Dopamine receptor blocking agents |
|
(i) Phenothiazines- Chlopromazine, Prochloperazine, Thioridazine, Trifluoperazine |
|
(ii) Butyrophenones- Haloperidol, Pimozide |
|
(iii) Benzamides- Metoclopramide, Clebopride |
|
2. Dopamine depleting agents – Reserpine, Alpha- methyldopa, Opiates |
|
3. Histamine receptor antagonist - Cimetidine, Ranitidine |
|
4. Serotonin reuptake inhibitors- Fluoxetine |
|
5. Stimulator of serotonergic pathway- Amphetamine and Hallucinogens |
|
6. Estrogens, Antiandrogens. |
|
7. Calcium channel blockers- Verapamil |
29.6 Clinical Presentation
The predominant physiological consequence of hyperprolactinaemia is suppression of pulsatile GnRH. The clinical manifestations of conditions vary significantly depending on the age and the sex of the patient and the magnitude of prolactin excess. Clinical presentation in women is more obvious and occurs earlier than in men (Table 29.4). Presenting symptoms in women are manifold and range from those arising due to hypogonadism (oligo-ovulation, anovulation, menstrual irregularities, symptoms of hypo-oestrogenism) to those that occur by lactotroph stimulation of breasts causing galactorrhoea. In addition, these women can also present with neurological symptoms caused by mass effects of the tumour within or around the pituitary. Symptoms include headache, visual field loss, cranial neuropathy, hypo-pituitarism, seizures and cerebrospinal fluid rhinorrhoea [16].
Table 29.4
Clinical presentation in female
|
Delayed puberty |
|
Amenorrhea |
|
Oligomenorrhea |
|
Luteal phase defects |
|
Infertility |
|
Galactorrhea |
|
Decreased libido |
|
Decreased bone mass density |
|
Signs of chronic hyperandrogenism |
|
Symptoms of hypothyroidism |
|
Symptoms related to pitutary adenoma |
It is worth noting that many women with hyperprolactinaemia do not have galactorrhoea, and many with galactorrhoea do not have hyperprolactinaemia. This is because galactorrhoea requires adequate oestrogen or progesterone priming of breasts. Conversely, isolated galactorrhoea with normal prolactin levels occurs due to increased sensitivity of the breast to the lactotrophic stimulus [17].
Approximately 3–10 % women with PCOS have co-existent hyperprolactinaemia [18]. Ovulatory dysfunction and hyper-androgenism both are commonly existing clinical presentations in patients with hyperprolactinaemia as well as in PCOS. Persistently elevated oestrogen levels are often found in women with PCOS which could result in prolactin elevation. However, it is still controversial whether they have cause-effect relationship or share a common mechanism or it is just a coincidental finding.
Prolonged hypo-oestrogenism secondary to hyperprolactinaemia may result in osteopenia [19]. Spinal bone mineral density (BMD) is decreased by approximately 25 % in such women and is not necessarily restored with normalization of prolactin levels [20, 21]. Hyperprolactinaemic women may present with signs of chronic hyper-androgenism such as hirsutism and acne, possibly due to increased dehydro-epiandro-sterone sulfate secretion from the adrenals [22], as well as reduced sex hormone-binding globulin leading to high free testosterone levels.
29.7 Diagnostic Evaluation
29.7.1 Serum Prolactin Estimation
Prolactin is a very dynamic hormone, so caution must be taken while diagnosing women to have hyperprolactinaemia warranting treatment. Careful history of drug ingestion, any stressful condition preceding sample collection including history of sexual intercourse, breast stimulation and chest wall injury should be noted. Chronic renal disease and thyroid disorder also needs to be ruled out. Normal serum prolactin levels in females vary between 5 and 25 ng/ml, with physiological and diurnal variations [23]. Serum prolactin levels are higher in the afternoon and hence should preferably be measured in the fasting morning sample [24]. Hyperprolactinaemia is usually defined as fasting levels of above 20 ng/ml in men and above 25 ng/ml in women [8]. Unless the prolactin levels are markedly elevated (>50 ng/ml), the investigation should be repeated before labeling the patient as hyperprolactinaemic. Even one normal value should be considered as normal, and an isolated raised one should be discarded as spurious (Fig. 29.1).
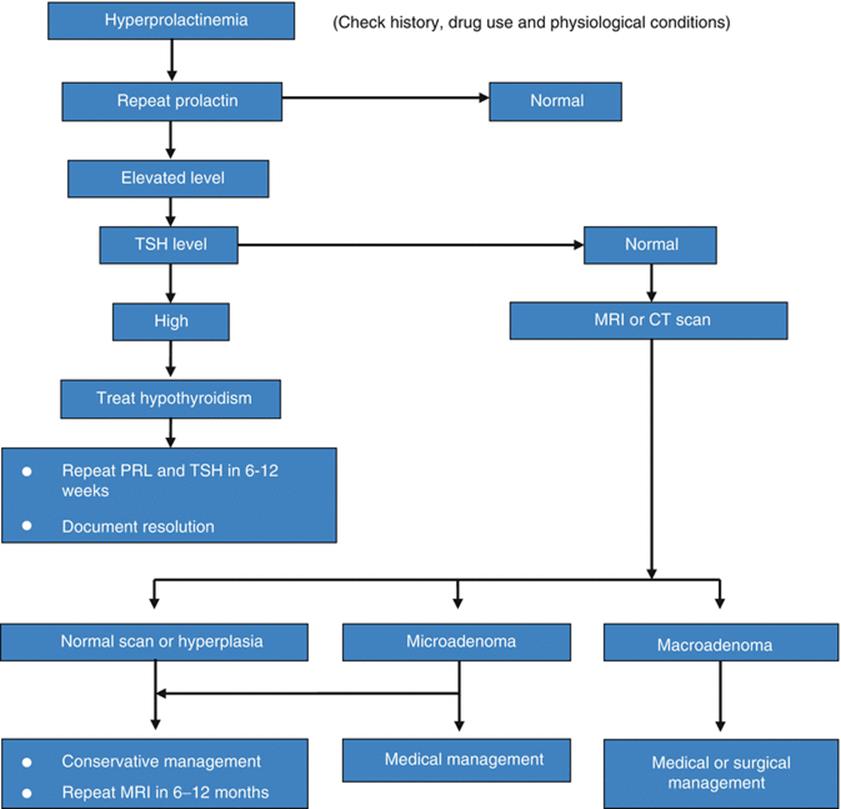
Fig. 29.1
Approach to management of patient with hyperprolactinemia
29.7.2 Radiological Imaging
This should only be advised judiciously to patients with confirmed diagnosis of hyperprolactinaemia. A mildly elevated serum prolactin level may be due to a non-functioning pituitary adenoma or craniopharyngioma compressing the pituitary stalk, but high prolactin levels are commonly associated with prolactinomas [2]. Increased use of CT scan and MRI may reveal other silent pituitary masses which neither pose any mass effect nor elevation of prolactin levels and are called ‘incidentaloma’ [25]. A prolactinoma is likely if the prolactin level is greater than 250 ng/ml [26], and a level of 500 ng/ml or greater is usually diagnostic of a macro-prolactinoma [1]. Drug intake usually elevates prolactin levels up to 100 ng/ml. But few drugs including risperidone and metoclopramide may cause prolactin elevations above 200 ng/ml [27]. In cases where other causes of hyperprolactinaemia have been excluded and no adenoma can be visualized with MRI, the hyperprolactinaemia is referred to as ‘idiopathic’ and should be treated on the merit of the symptoms caused or whether fertility is desired.
29.8 Treatment Approach
The management of hyperprolactinaemia should be individualized on the basis of clinical findings, underlying cause and the presence of hypogonadism or infertility. Asymptomatic women with hyperprolactinaemia and/or micro-adenomas, who are not concerned about fertility, may be followed without active intervention. Annual clinical review and prolactin assay might be sufficient if the clinical condition remains stable.
29.9 Treatment of Hyperprolactinaemia With Anovulatory Infertility
In the absence of other factors, anovulatory infertility due to hyperprolactinaemia is successfully treated with dopamine agonists. Exogenous gonadotropin stimulation can be added along with dopamine agonist to achieve optimal ovulation. However, if an underlying treatable cause is identified, it should be eliminated first.
29.9.1 Elimination of Known Cause
When the problem is drug induced, we should consider whether the medication can be discontinued or replaced with another agent. Medication should be discontinued if it is safe to do so and serum prolactin level repeated. If the drug is essential for the patient’s health (e.g. psychotropic agent) but is causing symptoms of hyperprolactinaemia, treatment with a dopamine agonist should be avoided, since it might compromise the effectiveness of the essential drug. Such patients should simply be treated with gonadotropins for the purpose of ovulation induction or replacement of sex steroids for hypogonadism.
Treatment of hypothyroidism with thyroid replacement therapy often restores the elevated prolactin level to normal. If the prolactin levels do not respond to adequate thyroid replacement, dopamine agonist may be required to treat the hyperprolactinaemia. If hyperprolactinaemia is associated with hypo-adrenalism, replacement treatment with corticosteroids lowers prolactin levels [28]. In case of a prolactin-secreting tumour, surgical therapies should be considered only when they are resistant to medical treatment or based on the symptoms caused due to their location or size [29].
29.9.2 Drug Therapy
29.9.2.1 Dopamine Agonist
Dopamine agonist is the mainstay of medical management and has revolutionized the treatment of idiopathic hyperprolactinaemia, as well as prolactinomas. Dopamine agonists have been in clinical use for many years and remain the cornerstone for therapy of hyperprolactinaemia [1, 30]. Most commonly used dopamine agonists are bromocriptine and cabergoline. Others are lisuride, pergolide, quinagolide, terguride and metergoline. Patients who are intolerant or fail to respond to one agent may do well with another.
In patients with idiopathic and micro-adenoma-associated hyperprolactinaemia, prolactin levels may reduce in a week, but ovulation and menstruation require few weeks to normalize. Weekly assessment of progesterone is the most popular method to confirm resumption of ovulatory function. Restoration of prolactin levels to normal after using dopamine agonist results in ovulation with a pregnancy rate of approximately 70 % [31].
Bromocriptine
Bromocriptine is a lysergic acid derivative with a bromine substitute at position 2 [32]. It is a strong dopamine agonist, binds to dopamine receptor and inhibits prolactin secretion. It decreases prolactin synthesis, DNA synthesis, cell multiplication and overall size of prolactinoma. It has a short half-life and so requires 2–3 times daily administration to maintain optimal suppression of prolactin levels. A daily dose of 5.0 mg is effective in about two thirds of the cases, but to save time one can commence with 7.5 mg/day in divided doses. Only 10 % of cases will need a higher dose than that, but it is usually ineffective to raise the dose above 20–30 mg/day. The drug may also cause mild drowsiness, hence one should avoid taking it prior to driving and preferably take it before sleep.
Intolerance to bromocriptine is common and the main indication of using an alternative drug. To avoid intolerance, it may be better to start with the lowest possible dose of 1.25 mg/day in the evening with food. If side effects are not too troublesome, a second dose of 1.25 mg with food in the morning is added next week. Thus, we gradually increase the dose by 1.25–2.5 mg/day each week till optimal dose is reached. Serum progesterone should be estimated weekly or in luteal phase periodically to check ovulation.
Vaginal usage of the same drug is better tolerable and causes lesser gastritis, nausea as well as sedation. Vaginal absorption is nearly complete, and avoidance of the liver first-pass metabolism allows lower therapeutic dosing [33]. Studies are also there regarding efficacy and safety of its long-acting form (depot-bromocriptine and slow release forms) [34, 35], but because of the availability of better tolerable drugs like cabergoline, these forms are not in routine clinical use. Bromocriptine has good treatment results, but the problem is that prolactin returns to elevated levels in 75 % of patients after discontinuation of treatment and there is no clinical or laboratory assessment that can predict those patients who will have long-term beneficial results [36].
Side effects associated with this drug are nausea, vomiting, headache, constipation, dizziness, faintness, depression, postural hypotension, digital vasospasm and nasal stuffiness. These symptoms are most likely to occur with initiation of treatment or when the dose is increased. One rare but notable side effect is neuropsychiatric symptoms like auditory hallucinations, delusion and mood changes. This may be due to hydrolysis of the lysergic acid part of the molecule. It quickly resolves with discontinuation of drug [37].
Cabergoline
Cabergoline shares many characteristics and adverse effects of bromocriptine but has a very long half-life allowing weekly dosing. This is more effective in suppressing prolactin levels and reducing tumour size [38]. The lower incidence of side effects and the weekly dosage makes cabergoline a better choice for initial treatment. It can also be given vaginally if intolerable in oral administration [39]. A dose of 0.25 mg twice per week is usually adequate for hyperprolactinaemia. Maximum dose that can be given is 1 mg twice a week. Once pregnancy is established, one can discontinue the dopamine agonist.
29.9.2.2 Ovulation-Inducing Agents
Pulsatile Gonadotropin-Releasing Hormone (GnRH) Therapy
As the main cause of anovulation is impaired gonadotropin pulsatility and derangement of the oestrogen-positive feedback effect on LH secretion, so pulsatile GnRH therapy combined with human chorionic gonadotropin (hCG) can be used in these patients [11]. Administration of GnRH in pulsatile mode restores the periodic release of FSH and LH from the pituitary, which corrects anovulation.
It is administered by a computerized mini-pump via a chronic indwelling intravenous or subcutaneous catheter. Subcutaneous route is preferred for its convenience and lack of invasiveness, but intravenous administration yields more predictable response and a higher rate of ovulatory cycle. Lower dose should be used initially in order to minimize the likelihood of multiple pregnancies due to hyper-stimulation of the ovaries. Pulsatile GnRH administration may be discontinued after ovulation, and the corpus luteum is supported by exogenous hCG. Adverse effects include infection and haematoma at cannula site, antibody formation, hyper-stimulation and rarely desensitization due to inadequate dose or frequency [40].
Human Gonadotropins as Substitution Therapy
If hyperprolactinaemia does not respond to treatment with dopamine agonist alone to cause ovulation or a patient is unable to tolerate the doses required to suppress high prolactin levels effectively, ovulation induction can be done by gonadotropins.
Gonadotropins used for ovulation induction in women are either urinary or recombinant products. If a patient has amenorrhoea, it is similar to that of hypogonadotropic hypogonadism; therefore, effective folliculogenesis and steroidogenesis require administration of combination of both FSH and LH. Human menopausal gonadotropin, which is derived from postmenopausal urine, contains both LH and FSH in a ratio of 1:1 and appears appropriate to be used for such cases. However, patients with oligomenorrhoea can also be treated with recombinant FSH alone as some LH continues to be secreted in such women. Widespread availability, safety and consistency of recombinant gonadotropin make it more suitable for ovulation induction. Ovarian stimulation is started with gonadotropins, and the response is monitored by ultrasound along with rising serum oestradiol levels. When a dominant follicle attains a size of 18 mm, hCG is given to promote final stage of oocyte maturation and ovulation. Post-ovulatory luteal support is mandatory till pregnancy is achieved and continued for the first 8–9 weeks of gestation. However, if menstruation ensues, luteal support is discontinued and a fresh cycle of gonadotropin stimulation can be planned. In these cases which are treated with gonadotropins rather than dopamine agonist, high prolactin levels may coexist.
Clomiphene Citrate
After complete normalization of prolactin levels with dopamine agonist with no evidence of ovulation by weekly estimation of progesterone in the following 6–8 weeks, it is important to rule out underlying PCOS. These patients often show polycystic ovaries with increasing oestradiol levels and can be effectively treated by combining clomiphene citrate to the dopamine agonist.
29.9.3 Surgical Excision of Prolactinomas
Response with medicines is variable as some prolactinomas show prompt shrinkage with low dose, while others may require prolonged treatment with higher dosage [14, 41]. Even in macro-prolactinomas, surgery is reserved for refractory and medication-intolerant patients [42]. Common indications of surgery are very large tumours, those with suprasellar and frontal extension, major chiasmal compression and visual impairment persisting after medications. Besides the usual surgical risks, hypo-pituitarism is a potential long-term effect of surgery and should be discussed with patients as part of the decision-making process. Unfortunately, excision is often incomplete and therefore relapse occurs although prolactin levels are lower than before.
29.9.4 External Radiation Therapy
External radiation therapy is only reserved for residual tumour in patients who have undergone surgery. It is of very limited benefit in the treatment of these patients, since the response is typically quite modest and delayed [43]. Patients should be counseled that such treatment carries a risk of developing hypo-pituitarism.
29.10 Pregnancy and Lactation
In idiopathic hyperprolactinaemia, dopamine agonist is stopped as soon as pregnancy is confirmed. As the risk of tumour expansion is low in micro-adenomas during pregnancy, dopamine-agonist therapy is stopped even in these cases. In cases of macro-prolactinoma, it is essential to monitor visual fields from time to time during pregnancy to detect tumour expansion, and if it is suspected, MRI can confirm the need for medical or surgical intervention. Serial prolactin assays during pregnancy are not helpful in predicting tumour expansion during gestation [44]. If symptomatic tumour enlargement (headache, visual field defect or diabetes insipidus) is detected, the treatment of choice is to reinstitute dopamine agonist [29, 45] with monthly follow-up. Though both drugs have been found to be safe in pregnancy, the number of reports studying bromocriptine’s use in pregnancy far exceeds that of cabergoline. Indications for surgery during pregnancy are severe chiasmal compression by a tumour unresponsive to drug therapy and the rare occurrence of pituitary apoplexy. Whenever possible, defer the operation until after delivery. Breastfeeding does not pose any demonstrable risk to the mother with a prolactinoma.
29.11 Conclusion
Prolactin is a very dynamic hormone and eludes diagnosis because of multiple physiological factors interfering in its estimation. Therefore, it is pertinent to establish the pathological relevance of hyperprolactinaemia as the main cause of ovulatory dysfunction. Dopamine agonist is the mainstay of treating anovulatory hyperprolactinaemia. Gonadotropins can be used as substitution therapy to induce ovulation in women who do not respond to or tolerate dopamine agonist. However, if the underlying cause of hyperprolactinaemia warrants treatment on its own merit, it needs to be addressed separately.
References
1.
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA, Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273–88.CrossRefPubMed
2.
Biller BM, Luciano A, Crosignani PG, Molitch M, Olive D, Rebar R, et al. Guidelines for the diagnosis and treatment of hyperprolactinemia. J Reprod Med. 1999;44 Suppl 12:1075–84.PubMed
3.
Schlesselman JJ. How does one assess the risk of abnormalities from human in vitro fertilization? Am J Obstet Gynecol. 1979;135(1):135–48.PubMed
4.
Vleugels MP, Eling WM, Rolland R, de Graaf R. Cortisol levels in human pregnancy in relation to parity and age. Am J Obstet Gynecol. 1986;155(1):118–21.CrossRefPubMed
5.
Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–631.PubMed
6.
Gibney J, Smith TP, McKenna TJ. The impact on clinical practice of routine screening for macroprolactin. J Clin Endocrinol Metab. 2005;90(7):3927–32.CrossRefPubMed
7.
Benker G, Jaspers C, Häusler G, Reinwein D. Control of prolactin secretion. Klin Wochenschr. 1990;68(23):1157–67.CrossRefPubMed
8.
Halbreich U, Kinon BJ, Gilmore JA, Kahn LS. Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinology. 2003;28 Suppl 1:53–67.CrossRefPubMed
9.
Nilsson LA, Roepstorff C, Kiens B, Billig H, Ling C. Prolactin suppresses malonyl-CoA concentration in human adipose tissue. Horm Metab Res. 2009;41(10):747–51.CrossRefPubMed
10.
Torre DL, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag. 2007;3(5):929–51.PubMedCentralPubMed
11.
Matsuzaki T, Azuma K, Irahara M, Yasui T, Aono T. Mechanism of anovulation in hyperprolactinemic amenorrhea determined by pulsatile gonadotropin-releasing hormone injection combined with human chorionic gonadotropin. Fertil Steril. 1994;62(6):1143–9.PubMed
12.
Martikainen H, Rönnberg L, Puistola U, Tapanainen J, Orava M, Kauppila A. Prolactin suppression by bromocriptine stimulates aromatization of testosterone to estradiol in women. Fertil Steril. 1989;52(1):51–4.PubMed
13.
Rao AR. Hyperprolactinemia and thyroid disorders. In: Rao KR, Carp H, Fischer R, editors. Principles and practice of assisted reproductive technology. New Delhi: Jaypee Brothers Medical Publishers Ltd; 2014. p. 127–37.
14.
Webster J, Scanlon MF. Prolactinomas. In: Sheaves R, Jenkins PJ, Wass JA, editors. Clinical endocrine oncology. 1st ed. Oxford: Blackwell Science; 1997. p. 189–94.
15.
Huang I, Gibson M, Peterson CM. Endocrine disorders. In: Berek JS, editor. Berek and Novak’s gynecology. 14th ed. Philadelphia: Lippincott Williams and Wilkins; 2007. p. 1069–136.
16.
Luciano AA. Clinical presentation of hyperprolactinemia. J Reprod Med. 1999;44 Suppl 12:1085–90.PubMed
17.
Boyd 3rd AE, Reichlin S, Turksoy RN. Galactorrhea-amenorrhea syndrome: diagnosis and therapy. Ann Intern Med. 1977;87(2):165–75.CrossRefPubMed
18.
Minakami H, Abe N, Oka N, Kimura K, Tamura T, Tamada T. Prolactin release in polycystic ovarian syndrome. Endocrinol Jpn. 1988;35:303–10.CrossRefPubMed
19.
Klibanski A, Neer RM, Beitins IZ, Ridgway EC, Zervas NT, McArthur JW. Decreased bone density in hyperprolactinemic women. N Engl J Med. 1980;303:1511–4.CrossRefPubMed
20.
Schlechte J, el-Khoury G, Kathol M, Walkner F. Forearm and vertebral bone mineral in treated and untreated hyperprolactinemic amenorrhea. J Clin Endocrinol Metab. 1987;64(5):1021–6.CrossRefPubMed
21.
Klibanski A, Biller BM, Rosenthal DI, Schoenfeld DA, Saxe V. Effects of prolactin and estrogen deficiency in amenorrheic bone loss. J Clin Endocrinol Metab. 1988;67(1):124–30.CrossRefPubMed
22.
Biller BM. Hyperprolactinemia. Int J Fertil Womens Med. 1999;44(2):74–7.PubMed
23.
Melmed S, Jameson JL. Disorders of the anterior pituitary and hypothalamus. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s principles of internal medicine. 16th ed. New York: McGraw Hill; 2008. p. 2076–97.
24.
Halvorson ML. Reproductive endocrinology. In: Hoffman BL, Schorge JO, Schaffer JI, Halvorson ML, Bradshaw KD, Cunningham FG, editors. Williams gynecology. 2nd ed. New York: McGraw Hill; 2012. p. 400–39.
25.
Burrow GN, Wortzman G, Rewcastle NB, Holgate RC, Kovacs K. Microadenomas of the pituitary and abnormal sellar tomograms in an unselected autopsy series. N Engl J Med. 1981;304(3):156–8.CrossRefPubMed
26.
Erem C, Kocak M, Nuhoglu I, Yılmaz M, Ucuncu O. Blood coagulation, fibrinolysis and lipid profile in patients with prolactinoma. Clin Endocrinol (Oxf). 2010;73(4):502–7.
27.
Kearns AE, Goff DC, Hayden DL, Daniels GH. Risperidone-associated hyperprolactinemia. Endocr Pract. 2000;6(6):425–9.CrossRefPubMed
28.
Donadio F, Barbieri A, Angioni R, Mantovani G, Beck-Peccoz P, Spada A, et al. Patients with macroprolactinaemia: Clinical and radiological features. Eur J Clin Invest. 2007;37(7):552–7.CrossRefPubMed
29.
Doody KJ. Treatment of infertile couple. In: Hoffman BL, Schorge JO, Schaffer JI, Halvorson ML, Bradshaw KD, Cunningham FG, editors. Williams gynecology. 2nd ed. New York: McGraw Hill; 2012. p. 529–53.
30.
Schlechte JA. Long-term management of prolactinomas. J Clin Endocrinol Metab. 2007;92(8):2861–5.CrossRefPubMed
31.
Randeva HS, Davis M, Prelevic GM. Prolactinoma and pregnancy. BJOG. 2000;107(9):1064–8.CrossRefPubMed
32.
Vance ML, Evans WS, Thorner MO. Drugs five years later. Bromocriptine. Ann Intern Med. 1984;100(1):78–91.CrossRefPubMed
33.
Katz E, Weiss BE, Hassell A, Schran HF, Adashi EY. Increased circulating levels of bromocriptine after vaginal compared with oral administration. Fertil Steril. 1991;55(5):882–4.PubMed
34.
Merola B, Colao A, Caruso E, Sarnacchiaro F, Briganti F, Lancranjan I, et al. Oral and injectable long-lasting bromocriptine preparations in hyperprolactinemia: comparison of their prolactin lowering activity, tolerability and safety. Gynecol Endocrinol. 1991;5(4):267–76.CrossRefPubMed
35.
Brue T, Lancranjan I, Louvet JP, Dewailly D, Roger P, Jaquet P. A long-acting repeatable form of bromocriptine as long-term treatment of prolactin-secreting macroadenomas: a multicenter study. Fertil Steril. 1992;57(1):74–80.PubMed
36.
Passos VQ, Souza JJ, Musolino NR, Bronstein MD. Long-term follow-up of prolactinomas: normoprolactinemia after bromocriptine withdrawal. J Clin Endocrinol Metab. 2002;87(8):3578–82.CrossRefPubMed
37.
Turner TH, Cookson JC, Wass JA, Drury PL, Price PA, Besser GM. Psychotic reactions during treatment of pituitary tumours with dopamine agonists. Br Med J (Clin Res Ed). 1984;289(6452):1101–3.CrossRef
38.
Di Sarno A, Landi ML, Cappabianca P, Di Salle F, Rossi FW, Pivonello R, et al. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001;86(11):5256–61.CrossRefPubMed
39.
Motta T, de Vincentiis S, Marchini M, Colombo N, D’Alberton A. Vaginal cabergoline in the treatment of hyperprolactinemic patients intolerant to oral dopaminergics. Fertil Steril. 1996;65(2):440–2.PubMed
40.
Shoham Z, Homburg R, Jacobs HS. Induction of ovulation with pulsatile GnRH. In: Crosignani PG, editor. Clinical obstetrics and gynaecology. London: Balliere Tindall WB Saunders Publication; 1990. p. 589–608.
41.
Essaïs O, Bouguerra R, Hamzaoui J, Marrakchi Z, Hadjri S, Chamakhi S, et al. Efficacy and safety of bromocriptine in the treatment of macroprolactinomas. Ann Endocrinol (Paris). 2002;63(6 Pt 1):524–31.
42.
Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65(2):265–73.CrossRef
43.
Tsagarakis S, Grossman A, Plowman PN, Jones AE, Touzel R, Rees LH, et al. Megavoltage pituitary irradiation in the management of prolactinomas: long-term follow-up. Clin Endocrinol (Oxf). 1991;34(5):399–406.CrossRef
44.
Molitch ME. Pregnancy and the hyperprolactinemic woman. N Engl J Med. 1985;312(21):1364–70.CrossRefPubMed
45.
Gemzell C, Wang CF. Outcome of pregnancy in women with pituitary adenoma. Fertil Steril. 1979;31(4):363–72.PubMed