Margaret Stanley1
(1)
Department of Pathology, University of Cambridge, Tennis Court Road, Cambridge, CB2 1QP, UK
Margaret Stanley
Email: mas1001@cam.ac.uk
Introduction
Papillomaviruses are small double stranded DNA viruses infecting the squamous epithelia (skin and internal mucosae) of both animals and man. Human papilloma viruses (HPVs) are not classified as serotypes but as genotypes on the basis of the DNA sequence of the major coat protein L1. HPVs are a very large branch of the papillomavirus family with the DNA of more than 130 HPV types, many of which have been fully sequenced, having been isolated from tissue biopsies (de Villiers 2013). Despite the daunting number of HPVs they fall basically into two groups: those that infect skin, or cutaneous surfaces, and those that infect the internal wet squamous mucosal surfaces, particularly the genital tract. Within these groups there are low-risk types (LRHPV), which generate benign lesions, in other words warts, and high-risk or oncogenic types (HRHPV), that are associated with cancers and their precursor lesions.
Burden of Disease Attributed to Genital HPVs
Approximately 40 HPV types regularly or sporadically infect the mucosal epithelial surfaces of the lower genital tract causing both warts and cancers. The LRHPV types, HPV6 and 11 cause more than 90 % of external genital warts with minor types (HPV42,44) and some HRHPVs contributing to the remaining 10 % (Lacey et al. 2006). HPV associated malignant disease in the genital tract is dominated by HPV16 and HPV18 which, with their close relatives, 31, 33, 35, 52, 58, 39, 45, 59, 56, 66 and 51, are the cause of virtually all cervical cancer and the majority of the high grade cervical cancer precursor lesions CIN2/3 (cervical intra-epithelial neoplasia grades 2 and 3) (Bosch et al. 2008). Thus 99 % or more of biopsies of invasive cervical cancer worldwide, and approximately 80 % of CIN 2/3 contain HRHPV DNA sequences. HPV16 dominates, and is present in at least 50 % of cancers irrespective of geographical location, followed by HPV18, 7–20 %. However invasive cervical cancer is not the only malignant disease associated with HRHPV infection, HPV DNA sequences are found in most anal and tonsil carcinomas, and a proportion of vulvar, vaginal, penile and head and neck cancers. HPV16 again is the dominant oncogenic type (Parkin and Bray 2006; Gillison et al. 2012). Overall, the global malignant burden attributable to HPV infection is calculated to be 5.2 % of all cancers (Ferlay et al. 2010).
Benign disease caused by the mucosal LRHPVs is not trivial. Genital warts are the most common viral sexually transmitted disease, they are highly infectious, refractory to therapy, result in significant morbidity and are a health economic burden (Woodhall et al. 2009). In immunocompromised patients, such as those with HIV they can form large polypoid growths requiring surgical intervention and be a clinical management problem (Denny et al. 2012). A maternal history of genital warts (Silverberg et al. 2003) is associated with a 231-fold risk for recurrent respiratory papillomatosis (RRP) an uncommon but potentially devastating disease, characterized by the growth of wart-like benign neoplasms throughout the aerodigestive tract that often requires repeated surgeries (Derkay and Darrow 2000).
HPV Vaccines: Rationale
Traditionally prophylactic vaccines that generate virus specific neutralising antibody have represented a cost effective means to control viral diseases. HPV should, in theory, be no exception but the exquisite host and tissue tropism and complex biology of the papillomaviruses differentiates them from most other viruses against which vaccination has proved successful. The HPV life cycle is exclusively intra-epithelial and only a fully differentiated squamous epithelium supports the complete infectious cycle and the production of infectious particles (Doorbar et al. 2012). There is no detectable viraemia, virus particles are shed from mucosal surfaces far from lymphatics and vascular channels and, not surprisingly, systemic cellular and humoral immune responses to HPV antigens are poor (Stanley 2008). Serum neutralizing antibody to the major capsid protein L1 is generated in genital HPV infections but neutralizing antibody titres are very low and only about 50–70 % of infected individuals sero-convert (Carter et al. 2000). Furthermore the degree of protection and the duration afforded by antibody in natural infections is not known. Re-infection with the same HPV genotype and reactivation of latent virus is thought to occur, even in seropositive individuals, so would vaccines that generate neutralising antibody protect against HPV infection and disease?
The evidence from animal papillomavirus infections, including some of the earliest published works from Shope, the founding father of papillomavirus research, showed very clearly that neutralizing antibody was protective (Shope 1937). In Shope’s experiments if rabbits were infected systemically with the cotton tail rabbit papillomavirus (CRPV) by direct injection of virus into the muscle or bloodstream, papillomas did not arise on the skin of the animals but neutralizing antibody was generated and the animals were completely resistant to viral challenge by abrasion of the skin, the route of infection by papillomaviruses. This and other data suggested very strongly that generating neutralizing antibody to virus coat protein would be an effective prophylactic vaccine strategy. Neutralising antibodies are directed against the L1 coat or capsid protein and the generation of this antibody requires the protein to be in the tertiary or native form. Since these viruses cannot be grown in bulk in tissue culture and viral particles particularly of the oncogenic types are sparse in lesions, the generation of native, or properly folded L1 protein, was challenging. The challenge was met by the demonstration that if the L1 gene was expressed via a viral or yeast vector, the L1 protein was produced in large amounts and self-assembled into a macromolecular structure, a virus-like particle (VLP) an empty capsid that is geometrically and antigenically almost identical to the native virion (Zhou et al. 1991; Kirnbauer et al. 1992). These VLPs were shown to generate neutralizing antibody in the animal models and immunized animals were protected against high-level virus challenge (Suzich et al. 1995; Breitburd et al. 1995).
Licensed Prophylactic HPV Vaccines
The currently licensed prophylactic HPV vaccines are subunit vaccines comprised of virus-like particles (VLPs) formed of the L1 protein. Vaccine HPV VLPs are made using sophisticated recombinant technologies in which the L1 gene of specific HPV types is recombined into the host genome of the yeast Saccharomyces cerevisiae or the insect virus baculovirus and the L1 protein expressed via these recombinant vectors. The chemistry of the expressed protein is such that it spontaneously assembles into VLPs that are morphologically and antigenically similar to the wild-type virus particle illustrated in Fig. 9.1. However, VLPs lack DNA and are non-infectious and non-oncogenic (Stanley et al. 2006). There are two prophylactic HPV L1 VLP vaccines. These are Cervarix®, a bivalent HPV (bHPV) 16/18 product from GlaxoSmithKline Biologicals Rixensart, Belgium and Gardasil® (also known as Silgard), a quadrivalent HPV (qHPV) 6, 11, 16, 18 product from MSD, Whitehouse Station, New Jersey, USA. These products are licensed in more than 160 countries.
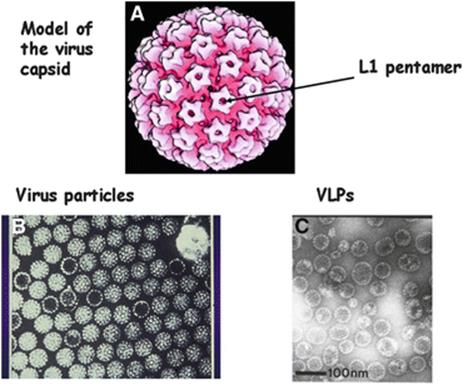
Fig. 9.1
Panel A: A model of the papillomavirus coat or capsid. There are two coat proteins L1 and L2. The rosette like surface structures (arrowed) are pentamers each consisting of five molecules of L1, one molecule of L2 fits into the central dimple of each pentamer. Panel B: Papillomavirus particles, both full (contain DNA) and empty particles can be seen. Panel C: HPV 16 L1 virus like particles, VLPs, made by expressing the HPV 16 L1 gene in baculovirus. The L1 protein so expressed spontaneously assembles into empty capsids or VLPs that are morphologically similar to the empty virus particles seen in panel B. From Stanley et al. 2006 with permission
Vaccine Efficacy
Both vaccines have undergone large, randomised, placebo controlled, double blind phase III trials (RCTs) in young women, 15–26 years old. For a detailed review see Schiller (Schiller et al. 2012) and also details in Table 9.1 and mentioned below.
Table 9.1
Phase III randomised control trials (RCTs) end of study: per protocol efficacy populations
|
Vaccine |
Quadrivalent |
Bivalent |
|
Women 15–26 years |
||
|
Mean follow up |
42 months |
42 months |
|
Prophylactic efficacy |
% 95 % CI |
% 95 % CI |
|
HPV16/18 CINa2 |
100 (95,100) |
95 (88,98) |
|
HPV16/18 CIN3 |
97 (88,100) |
92 (67,91) |
|
HPV 16/18 AISb |
100 (31,100) |
100 (−8,100) |
|
HPV16/18 VIN3c/VaIN3d |
100 (83,100) |
Not reported |
|
HPV6/11/16/18 VIN1/VaIN1 |
100 (86,100) |
Not a target |
|
HPV6/11/16/18 EGLe |
99 (97,100) |
Not a target |
|
Women 25–45 years |
||
|
6/11/16/18 PIf/CIN/VIN/VaIN |
89 (78,95) |
|
|
Men 16–23 years |
||
|
HPV 16/18/6/11 EGL (MSWg) |
90 (69,98) |
No studies |
|
HPV 16/18/6/11/AINh (MSM)i |
78 (40,93) |
No studies |
Notes
aCIN cervical intra-epithelial neoplasia
bAIS adenocarcinoma in situ of the cervix
cVIN vulval intra-epithelial neoplasia
dVaIN vaginal intra-epithelial neoplasia
eEGL external genital lesions
fPI persistent infection
gMSW men who have sex with women
hAIN anal intra-epithelial neoplasia
iMSM men who have sex with men
Quadrivalent vaccine: Data for CIN2/3 and AIS are a combined analysis of four randomised clinical trials (Kjaer et al. 2009). 20,583 women aged 16–23 years with a lifetime history of not more than 4–5 sex partners and negative for HPV 16 and/or 18 infection from month 0 though to 1 month post the 3rd immunisation were randomised to receive vaccine or placebo. The primary composite endpoint was the combined incidence of HPV 16/18 related CIN2/3, AIS or cervical cancer. Case definition was a diagnosis by the pathology panel of CIN2, CIN 3, AIS or invasive cervical cancer. Data for HPV 6/11/16/18 related anogenital disease from 5,455 women 16–24 years randomised to receive vaccine or placebo PCR and seronegative for vaccine type HPV at month 0 through to month 7. Coprimary composite endpoints were the incidence of HPV 6/11/16/18 related external genital warts vulval and vaginal intraepithelial neoplasia and invasive cancer
Bivalent vaccine: The data are from the pivotal Phase III randomised control trial PATRICIA (Lehtinen et al. 2012) n = 7,338 vaccine group n = 7,305 placebo. The according to protocol cohort are women 15–25 years HPV 16 and 18 sero-negative and PCR negative at baseline and at month 6, with less than 6 lifetime sexual partners (Lehtinen et al. 2012). The primary endpoint was the incidence of HPV 16/18 related CIN2/3
These trials were designed primarily to demonstrate efficacy in preventing high grade cervical intraepithelial neoplasms (CIN2/3) and Adenocarcinoma in situ, (AIS) caused by infection related to the vaccine HPV types (both vaccines) or vaginal intra-epithelial neoplasia (VaIN) and vulval intra-epithelial neoplasms (VIN) and external genital warts (qHPV vaccine). Two phase III studies evaluated the qHPV vaccine (Future I and Future II) and two evaluated the bHPV vaccine (Patricia and the Costa Rica vaccine trial (CVT)). The Future I and II and Patricia trials were company sponsored multi-centre trials in Europe, the Americas and Asia Pacific. The CVT was a US Government sponsored community based trial in Guanacaste Province, Costa Rica; the primary endpoint in this trial was 12 month HPV 16/18 persistent infection, a prerequisite for development of CIN. The qHPV vaccine has also undergone trials in 16–23 year old men to determine efficacy against external genital warts in heterosexual men (Giuliano et al. 2011) and anal intraepithelial neoplasia in men who have sex with men (Palefsky et al. 2011).
End of study analyses of the pivotal phase III trials in young women have now been published and are summarised in Table 9.1. The design of the RCTs differs significantly for both vaccines in terms of population demographics, baseline inclusion criteria for case assignment, serological and DNA detection methods (Schiller et al. 2012). However both vaccines have shown very high efficacy – greater than 94 % – against HPV 16/18 caused high grade CIN2/3 – the endpoint accepted as the ethically acceptable proxy for vaccine efficacy against cervical cancer (Pagliusi and Teresa Aguado 2004). In the according to protocol (ATP) cohort i.e., women 15–26 years old, HPV DNA negative by Polymerase Chain Reaction and sero-negative for vaccine type HPVs at trial entry and after completion of the three dose immunisation regime, efficacy against intra-epithelial cervical disease of all grades caused by the HPV types in the vaccines was greater than 90 % (Kjaer et al. 2009; Lehtinen et al. 2012) and is maintained for at least a decade (Roteli-Martins et al. 2012b; Rowhani-Rahbar et al. 2009).
Implementation and Effectiveness
HPV vaccines are prophylactic, not therapeutic, preventing not treating infection and they are not effective in individuals with already established infections. Genital HPV infection is usually sexually transmitted and the most important risk period for acquisition of a genital HPV is soon after the onset of sexual activity (Winer et al. 2008). The average age of sexual debut varies widely between societies but to be assured that the vaccine recipients receive protection, young adolescents in the 9–14 year age group should be targeted. Immunisation before puberty with HPV vaccines is immunologically optimal and antibody responses 2× greater are achieved in 9–13 year old adolescents compared to 15–23 year old women (Giuliano et al. 2007; Reisinger et al. 2007). The recommendations for HPV vaccination in most countries – both in the developing and developed world – recognise this and are remarkably uniform in targeting 12–14 year olds as the primary group for immunisation (Dorleans et al. 2010; Garland et al. 2011). Catch up programmes are recommended in some countries but there is variability in the age of the catch up populations (Dorleans et al. 2010).
Vaccine Impact
At the time of writing HPV vaccination has been incorporated into the National Immunisation Programme in 62 countries covering all continents and evidence of the impact on disease and infection is becoming available. Some of the most striking effectiveness data come from Australia which commenced an HPV immunisation programme in April 2007 targeted to girls aged 12–13 years with a catch up programme over 2 years for 13–26 year old young women. The programme was funded by the Federal Government but delivered by the States and Territories through schools and for the older girls via clinics and GP practices. The quadrivalent vaccine was used and coverage rates (for the full three dose regime) of approximately 75 % were achieved for the school programme (up to 17 years) with lower rates in the older girls (Garland et al. 2011).
Disease Impact
Genital warts, more than 90 % of which are caused by HPV 6 and 11, have a short natural history with lesions appearing within 3–6 months of infection. To assess vaccine impact on genital wart incidence, patients attending eight sexual health centres for the first time between 2004 and 2011 (n = 8,770) in major Australian cities were surveyed (Read et al. 2011). Those with a diagnosis of new genital warts (n = 7,686) were stratified by age group (<21 years, 21–30, > 30 years) at the time of presentation (Ali et al. 2013). Over the post-vaccination period July 2007 to mid-2011, the incidence of genital warts fell by 92.6 % in women under 21 years, 72.6 % in women under 30 years but there was no decline in those over 30 years. In men who have sex with women (MSW) aged <21 years incidence of genital warts fell by 81.8 %, by 51.1 % in those 21–30 years and 15 % in those >30 years. No decline was observed in bisexual or homosexual men.
Denmark introduced vaccination for 12 year old girls in December 2009 and this was preceded by a catch up programme for 13–15 year old girls in August 2008. Coverage overall was 85 %. Following the introduction of vaccination, the incidence of genital warts in females decreased by 3 % annually but no decline was observed in males (Baandrup et al. 2013). The most dramatic falls were in the youngest cohorts of 16–17 year old girls where genital warts were almost eliminated (Baandrup et al. 2013).
The rationale for immunising only one gender (females) against a sexually transmitted infection is that this generates herd protection by blocking transmission to effectively protect the non-immunised men (Garnett 2005). This hypothesis is supported by the Australian results but not, at the present, by the Danish data. The reasons for this are not clear currently, but geography may be relevant. Denmark is a small country easily accessible to countries with populations with low HPV vaccine coverage. Australia is a huge island with high vaccine coverage and major population centres thousands of kilometres distant from potential contact with non-immunised young women.
Disease Impact: Cervical Abnormalities
Reductions in cervical cancer will only be seen in the long term – decades after vaccination – but reductions in precancerous lesions caused by vaccine HPV types should be detectable in the medium term. Reductions in cervical abnormalities have been observed following the Australian National Vaccination Programme. In a retrospective cohort analysis between April 2007 and December 2011, the effectiveness of the HPV vaccine against CIN1, CIN2, CIN3 and AIS (histologically diagnosed) was assessed in vaccinated and unvaccinated women in the state of Victoria (Gertig et al. 2013). Vaccine effectiveness was highest in the cohort vaccinated at the youngest age (less than 14 years) with 75 % reduction in any high grade histology (CIN2/3 or AIS) compared to 32 % in those vaccinated at 17 years. Overall the data from this study and from other Australian states (Crowe et al. 2014) indicates that in the vaccinated cohorts (age 12–26 years) high grade cervical abnormalities (CIN2/3, AIS) have decreased by about 48 %, a situation predicted by data from the earlier randomised controlled trials (Munoz et al. 2010).
Vaccine Impact: Reduction in Infection
A key outcome of vaccination should be a reduction in virus load in the population overall. In a study of women attending Family Planning Clinics in two centres in Australia the prevalence of HPV 6,11,16,18 DNA in cervical swabs fell from 28.7 % before vaccination to 6.7 % post vaccination (Tabrizi et al. 2012). In the USA, vaccine cervical HPV type prevalence in females 14–19 years old in the pre vaccine era (2003–2007) was 11.5 %, but post vaccine (2007–2010) this fell to 5.1 % (Markowitz et al. 2013). The UK used the bivalent HPV vaccine from 2008 to 2012. In Scotland women are called for cervical cancer screening from age 20 years and in a study determining cervical HPV prevalence in cervical smears from this cohort 3 years post vaccination, a significant reduction in HPV 16 prevalence was observed (Kavanagh et al. 2014). In England women are not called for screening until age 25 years, but HPV16/18 prevalence pre and post vaccination was assessed in a study using vulval/vaginal swabs collected for Chlamydia screening in sexually active 16–24 year old women (Mesher et al. 2013). In the 16–18 year age group who had an estimated vaccine coverage of 65 %, HPV16/18 prevalence fell from 19.1 % to 6.5 % after vaccination compared to no change in the 22–24 year old cohort who were not immunised (Mesher et al. 2013).
Overall, studies assessing the impact of routine use of both commercially available vaccines in several countries have shown a significant reduction in prevalence of vaccine HPV types in young women. Studies using the qHPV vaccine have shown a consistent decrease in genital warts and early evidence of a reduction in high grade cervical abnormalities. It can be concluded that so far the vaccines are doing their job reducing disease and infection.
Mechanism of Protection
The current assumption is that the protection afforded via these vaccines is antibody mediated since passive immunisation is protective in animal models (Suzich et al. 1995; Breitburd et al. 1995) but how can antibody in the serum protect against infection with a virus that is exclusively intra-epithelial? HPV infects cells in the basal layer of squamous epithelium at multiple sites in the anogenital tract – the cervical squamo-columnar junction, the ectocervix, the upper and lower epithelium of the vagina, multiple sites on the vulva, perianal and intra-anal epithelium, penile shaft and the scrotal skin (Doorbar et al. 2012). The cervical squamo-columnar junction is bathed in cervical mucus and it could be argued that passive transport of serum antibody into these secretions would be the mechanism of protection. However, this could not explain protection at the well keratinised surfaces of the vulva, penis and scrotal skin. Virus neutralising antibody prevents virus entry into cells and the questions therefore are: how does HPV infect the basal cell of stratified squamous epithelium and how do neutralising antibodies prevent this?
These questions have been addressed in a series of elegant experiments with a cervico vaginal model of infection using a surrogate virus or pseudovirion (Roberts et al. 2007). Pseudovirions are L1/L2 VLPs that have packaged DNA encoding a reporter protein such as an enzyme or a fluorescent protein, the expression of which allows the pseudovirion to be tracked in cells or tissues. These studies showed that epithelial micro-abrasion and wound healing were necessary for HPV infection of basal cells. Only micro-abrasion that resulted in the removal of the full thickness of the epithelium but retention of the epithelial basement membrane permitted infection of basal cells, since pseudovirions attached first to the basement membrane (BM) before finally entering a basal cell. This sequence of events provides a mechanistic explanation for antibody protection and potentially for the recall or anamnestic memory response. The micro wound from the epithelial denudation would result in an immediate serous exudate into the wound bed. This exudate would be rich in large plasma proteins including serum immunoglobulins together with phagocytes and immunocytes including B memory cells. Virus neutralisation and potentially a modest recall response would then ensue.
Duration of Protection
Immune Memory
Immune memory is the basis of successful vaccination and the evidence from the vaccine RCTs and post-vaccine immunosurveillance suggests that HPV vaccines induce robust memory. The antibody response after the three dose immunisation schedule follows the expected pattern (Villa et al. 2006; Harper et al. 2006). After each vaccine dose antibody levels increase until a peak antibody concentration 50–1,000 times greater than natural infection is achieved 1 month after the 3rd and final dose in the primary schedule. Antibody concentrations wane over the subsequent 12–18 months but then stabilise at a plateau level with geometric mean titres (GMTs) on average 10 times greater than in the placebo groups. This pattern is consistent with the notion of the generation of a large population of antibody secreting plasma cells after dose 3, with varying life spans but some with the ability to migrate to the bone marrow surviving as long lived plasma cells maintaining a low but constant antibody production. Serum neutralising antibody persists with GMTs about 10 times greater than natural infection for the 7–9 year duration of the published studies (Roteli-Martins et al. 2012a; Rowhani-Rahbar et al. 2012). Mathematical modelling predicts slow decay of antibody over a 30–50 year period and potentially, therefore, protection over that time. Both type specific and cross neutralising antibodies are generated by VLP vaccines although concentrations of cross neutralising species are on average 100 times lower than type specific (Einstein et al. 2011).
Antigen challenge at 60 months after the first dose with both vaccines results in a rapid and robust anamnestic or recall response with antibody levels rising within 3–5 days to levels greater than that achieved at peak in the initial immunisation schedule, demonstrating the presence of reactive memory B cells (Olsson et al. 2007; Moscicki et al. 2012). Collectively these data strongly imply that HPV VLP immunisation generates both components of the antibody memory response i.e. serological memory and reactive memory, a prerequisite for long term vaccine induced protection. However at the present there is no immune correlate, and no antibody concentration (or other immune measurement) has been defined that correlates with protection.
Safety
The safety profile of both vaccines was assessed extensively in the RCTs and by robust pharmacovigilance in the post licensure setting using both passive and active vaccine surveillance. Passive surveillance is voluntary reporting in daily practice by vaccinated persons (or others) and medical professionals to manufacturers, national surveillance systems such as the USA VAERS and Australian TGA databases or multinational databases, for example the WHO VIGIbase and EUVAX of the EU commission (Labadie 2011) Active surveillance is the implementation of systematic procedures to actively seek and identify clinically significant events that occur within a defined period and/or population and include large post-licensure studies sponsored by the manufacturer or national regulatory authorities (Gee et al. 2011; Bonanni et al. 2010).
The most commonly reported vaccine related adverse events (AE’s) are injection site reactions including pain, swelling, erythema, these are usually of short duration and resolve spontaneously (Slade et al. 2009;Angelo et al. 2014). Systemic AE’s, such as myalgia, fatigue, have been mild and self-limited (Harris et al. 2014). Post vaccination syncope has occurred and is considered to be a psychogenic reaction (Buttery et al. 2008) and it is recommended that after vaccination there is a 15 min observation period. Serious vaccine related AE’s such as anaphylaxis are very rare. In Ontario between 2007 and 2011 691,994 doses of the qHPV vaccine were distributed in a school based programme, two cases of anaphylaxis were reported but on review the reports of anaphylaxis did not meet the Brighton anaphylaxis definition (Harris et al. 2014). No associations with new onset chronic conditions such as auto-immune disease have been identified in large well conducted population based studies (Gold and McIntyre 2010; Chao and Jacobsen 2012)
The key challenge faced in pharmacovigilance is to distinguish real AE’s from background conditions that would occur regardless of vaccination. Studies providing population based data on incidence of potential adverse events prior to vaccination allow analysis of observed/expected rates in vaccinated populations (Siegrist et al. 2007; Callreus et al. 2009). In the UK the MHRA (Medicines Health and Regulatory Agency) has the responsibility for monitoring HPV vaccine safety by analysis of passive surveillance data communicated via the yellow card report scheme (http://yellowcard.mhra.gov.uk/). A central element to this is the use of statistical tools to identify safety signals. As an example, to 2012 about 30 % of reports AE’s after immunisation with Cervarix were related to nervous system disorders most of which, such a syncope, were psychogenic – the fear or anticipation of a needle injection. There were six reports of encephalitis and one of encephalitis lethargica but, given the expected background incidence of these conditions, this is consistent with chance not causality. No safety signals were identified in any analyses for neurologic, auto-immune or other disease states.
HPV vaccines are not recommended for administration during pregnancy but surveillance of pregnancy outcomes following inadvertent vaccination has revealed no adverse outcomes such as miscarriage, congenital abnormalities or premature labour beyond background rates. Both manufacturers have pregnancy registries monitoring outcomes in those inadvertently vaccinated (Dana et al. 2009).
HPV vaccines are now given to boys in the Australian National Immunisation programme and the safety profile parallels that observed in girls. The Global Advisory Committee on Vaccine Safety (GACVS) of WHO recently published a safety update on HPV vaccines and commented: In summary, 4 years after the last review of HPV vaccine safety and with more than 170 million doses distributed worldwide and more countries offering the vaccine through national immunization programs, the Committee continues to be reassured by the safety profile of the available products.
Alternative Dosage Schedules
In view of the overwhelming data on efficacy from the RCTs and the emerging data on population effectiveness, the focus of discussions about the current vaccines is no longer about efficacy but rather about implementation, access and affordability. In this context changing the dosage schedules has been a topic of discussion. HPV vaccines are delivered in three doses at 0, 1–2 and 6 months. In immunological terms this is a ‘prime, prime boost’ schedule with the extended period between dose 2 and 3 required for the generation after dose 3 of high concentrations of high affinity antibody and robust immune memory. Several studies have shown that the interval between doses 2 and 3 can be extended (but not reduced) to 12 and even 24 months (Lamontagne et al. 2013; Brown et al. 2012; Esposito et al. 2011). In many settings this flexibility is important for implementation and high uptake of the vaccines.
Antibody responses in young adolescents before or at the time of puberty are optimal with antibody titres twice those achieved in the 16–26 year old women in whom efficacy has been demonstrated in the RCTs (Giuliano et al. 2007). Studies have investigated the feasibility in the young adolescent cohort of changing from the three dose ‘prime, prime, boost’ to a two dose ‘prime, boost’ at 0 and 6 months (Dobson et al. 2013; Romanowski et al. 2011). The evidence for both vaccines from these studies is that in 9–14 year old girls two doses at 0 and 6 months, antibody responses (titres and avidity) are non-inferior over a 3 or 4 year period to those achieved after three doses in 16–26 year old women (Boxus et al. 2014; Dobson et al. 2013).
At their meeting in April 2014 the Strategic Advisory Group of Experts on Immunization (SAGE) of WHO considered HPV vaccine schedules and made the following recommendations:
SAGE reiterated the importance of providing human papillomavirus immunization to girls as early as necessary, i.e. in girls aged 9 to 13 years prior to sexual debut, based on local data and patterns of sexual activity. Upon review of the evidence, SAGE recommended a 2-dose schedule for girls, if vaccination is initiated prior to 15 years of age. A 3-dose schedule remains necessary if immunization is initiated after the girls’ 15th birthday. The recommended minimal interval between the 2 doses is 6 months. This interval may be extended to 12 months if this facilitates administration. A 3-dose schedule (i.e. at 0, 1–2, and 6 months) remains recommended for immunocompromised individuals, including those known to be HIV-infected.
Changing dosage schedules in the absence of robust data on duration of protection is a risk and when public health and regulatory authorities adopt or recommend alternatives to the licensed three dose regimens they need to address this question, make risk assessments based on the evidence and devise risk management strategies for worst case scenarios to minimise any impact on cancer prevention strategies and other immunisation programmes – there should always be a plan B.
Conclusions
Benign and malignant disease caused by HPV constitutes a global public health problem. Genital warts are the commonest viral sexually transmitted infection and 5 % of all cancers are HPV associated. The unfolding of the HPV story started in the 1970s with the recognition that HPVs are a large family of viruses that include types that cause cancer particularly cancer of the cervix, a disease that kills 250,000 women each year. It has resulted in the development of two prophylactic virus like particle (VLP) vaccines using sophisticated recombinant molecular techniques and protein expression. Both vaccines target infection by the oncogenic HPVs 16 and 18 and one also targets the low risk HPVs 6 and 11 that cause genital and laryngeal warts. These vaccines are now included in the national immunisation programmes in many countries, with young adolescent peri-pubertal girls the usual cohort for immunisation. Population effectiveness in women is now being demonstrated in those countries with high vaccine coverage. Since HPV associated cancers in men are increasing in incidence, an issue of contemporary debate is extending HPV vaccination to adolescent boys.
HPV VLP vaccines are highly immunogenic, generating serum neutralising antibody that persists for at least 9 years and a robust recall response at 60 months post vaccination. Protection against vaccine type associated disease and infection lasts for at least a decade and models predict more than 30 years protection. At present the assumption is that the protection achieved by these vaccines against HPV induced disease is mediated via serum neutralising IgG and this is consistent with what is known of the mechanism of HPV infection in the genital tract. Emerging evidence shows that very low antibody concentrations are protective but at the present there is no immune correlate of protection, disease prevention remains the only measure of the effectiveness of HPV vaccines.
Safety is of paramount importance for vaccines and all findings for HPV vaccines from randomised control trials, passive and active vaccine surveillance reporting systems are highly consistent in showing that the HPV vaccines have a good safety profile. Vaccine adverse events are local injection associated and of short duration. Systemic events are mild and self-limiting, but safety monitoring continues in long term follow up studies.
The HPV vaccine story is a remarkable story of scientific achievement, entrepreneurial drive and commercial and scientific interaction. HPV 16 and HPV 18 DNAs were cloned from cervical carcinoma biopsies in Harald zur Hausen’s laboratory in 1983 and 1984, starting the explosion in HPV molecular biology and epidemiology that showed unequivocally that oncogenic HPVs were the cause of cervical cancer. HPV VLPs were first made in 1991 and 1992 and prophylactic HPV VLP vaccines were first licensed in 2006–15 years later. By 2014 more than 160 million doses of these vaccines had been distributed and millions of girls and women (and now men) are and will be protected against HPV induced disease, a major public health achievement.
Frequently Asked Questions
· Will being vaccinated prevent all cases of cervical cancer?
No, the two vaccines protect against HPV 16 and 18, the types that cause at least 70 % of cancers worldwide. Vaccinated women are at much lower risk but should still go for routine screening if it is available.
· How effective are these vaccines?
Both vaccines in women and girls who are not already infected prevent more than 98 % of the HPV16/18 caused pre-cancers (e.g. CIN), the obligate precursors to HPV 16/18 caused cancers. The quadrivalent vaccine prevents >90 % of genital warts.
· Should women who are sexually active be vaccinated?
Both vaccines are most effective when delivered to girls before the onset of sexual activity. The vaccines prevent infection, they do not treat infection or disease. Sexually active women receive benefit from vaccination since they are protected from re-infection or new infections by the vaccine HPV types. There is evidence that vaccinated women adequately treated (complete lesion excision) for HPV associated cervical disease (CIN2/3) are at lower risk for disease recurrence or new disease in the genital tract (Joura et al. 2012).
· What should I do if my patient becomes pregnant after the start of the immunisation schedule?
Immunisation should be stopped until the patient has delivered her baby. Immunisation can then be resumed following the original schedule. Thus, if the patient received one dose, then resume with two doses 6 months apart, if she received two doses give a third dose in the post-natal period. Extending the interval between doses does not reduce immunogenicity. It is not recommended that pregnant women receive the vaccines. However, the evidence is that pregnant women who have received the vaccine have no more or less risk of pregnancy related adverse events such as miscarriage, congenital malformation, stillbirth, than non-vaccinated women. Vaccines can be given to lactating women.
· How safe are these vaccines, what about reports of deaths?
The main side effect of receiving the HPV vaccine is pain at the injection site, this usually resolves within 24 h. Some people experience local swelling and redness but again this is short lived. About 10 % feel dizzy or nauseous and about 18 % will faint. This is needle fear and the recommendation is that immunised subjects are observed and sit in the office or waiting room for about 15 min after immunisation before returning home.
Deaths have been reported but none of these has been shown to be related to the vaccine – they are coincidental to vaccination. It is important to remember that there is a background incidence of death from unknown causes in girls and young women irrespective of vaccination and therefore in view of the large numbers of girls being vaccinated deaths will occur because of this background rate. Each tragedy has to be investigated thoroughly and swiftly and the bereaved families kept at the centre of events and informed at every stage of the investigations. It must be remembered that the main side effect of the HPV vaccine is a sore arm, the main side effect of cervix cancer is death.
· Do I need to give a booster?
At the present the evidence is that protection in immuno-competent subjects remains for at least a decade, the longest follow up time of vaccinated women to date. Mathematical models plotting antibody decay suggest that protection will be maintained for at least 30 years without boosting. No boost is needed.
· What about HIV infected and other immunosuppressed subjects – are the vaccines effective?
Safety and immunogenicity of the vaccines has been evaluated in small studies in HIV infected children and adults. The vaccines have an acceptable safety profile and are immunogenic with comparable antibody titres to immunocompetent patients but the studies so far only have a short follow up and the persistence of antibody and duration of protection is not known for these patients.
References
Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, Fairley CK, Guy RJ (2013) Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ 346:f2032PubMedCrossRef
Angelo MG, David MP, Zima J, Baril L, Dubin G, Arellano F, Struyf F (2014) Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf. doi:10.1002/pds.3554[doi]
Baandrup L, Blomberg M, Dehlendorff C, Sand C, Andersen KK, Kjaer SK (2013) Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex Transm Dis 40(2):130–135. doi:10.1097/OLQ.0b013e31827bd66bPubMed
Bonanni P, Cohet C, Kjaer SK, Latham NB, Lambert PH, Reisinger K, Haupt RM (2010) A summary of the post-licensure surveillance initiatives for GARDASIL/SILGARD. Vaccine 28(30):4719–4730PubMedCrossRef
Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Munoz N (2008) Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 26(Suppl 10):K1–K16PubMedCrossRef
Boxus M, Lockman L, Fochesato M, Lorin C, Thomas F, Giannini SL (2014) Antibody avidity measurements in recipients of Cervarix vaccine following a two-dose schedule or the licensed three-dose schedule. Vaccine. doi:10.1016/j.vaccine.2014.04.005, S0264-410X(14)00507-6 [pii]PubMed
Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowey DR (1995) Immunization with virus-like particles from cotton tail rabbit papillomavirus (CRPV) can protect against experimentally CRPV infection. J Virol 69:3959–3963PubMedCentralPubMed
Brown B, Blas M, Cabral A, Carcamo C, Gravitt P, Halsey N (2012) Randomized trial of HPV4 vaccine assessing the response to HPV4 vaccine in two schedules among Peruvian female sex workers. Vaccine 30(13):2309–2314. doi:10.1016/j.vaccine.2012.01.058PubMedCentralPubMedCrossRef
Buttery JP, Madin S, Crawford NW, Elia S, La Vincente S, Hanieh S, Smith L, Bolam B (2008) Mass psychogenic response to human papillomavirus vaccination. Med J Aust 189(5):261–262PubMed
Callreus T, Svanstrom H, Nielsen NM, Poulsen S, Valentiner-Branth P, Hviid A (2009) Human papillomavirus immunisation of adolescent girls and anticipated reporting of immune-mediated adverse events. Vaccine 27(22):2954–2958PubMedCrossRef
Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA (2000) Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis 181(6):1911–1919PubMedCrossRef
Chao C, Jacobsen SJ (2012) Evaluation of autoimmune safety signal in observational vaccine safety studies. Hum Vaccin Immunother 8(9):1302–1304. doi:10.4161/hv.21268, 21268 [pii]PubMedCentralPubMedCrossRef
Crowe E, Pandeya N, Brotherton JM, Dobson AJ, Kisely S, Lambert SB, Whiteman DC (2014) Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 348:g1458PubMedCentralPubMedCrossRef
Dana A, Buchanan KM, Goss MA, Seminack MM, Shields KE, Korn S, Cunningham ML, Haupt RM (2009) Pregnancy outcomes from the pregnancy registry of a human papillomavirus type 6/11/16/18 vaccine. Obstet Gynecol 114(6):1170–1178PubMedCrossRef
Denny LA, Franceschi S, de Sanjose S, Heard I, Moscicki AB, Palefsky J (2012) Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine 30(Suppl 5):F168–F174. doi:10.1016/j.vaccine.2012.06.045PubMedCrossRef
Derkay CS, Darrow DH (2000) Recurrent respiratory papillomatosis of the larynx: current diagnosis and treatment. Otolaryngol Clin North Am 33(5):1127–1142PubMedCrossRef
de Villiers EM (2013) Cross-roads in the classification of papillomaviruses. Virology 445(1–2):2–10. doi:10.1016/j.virol.2013.04.023PubMedCrossRef
Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, Langley JM, Bettinger JA, Singer J, Money D, Miller D, Naus M, Marra F, Young E (2013) Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 309(17):1793–1802. doi:10.1001/jama.2013.1625, 1682939 [pii]PubMedCrossRef
Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA (2012) The biology and life-cycle of human papillomaviruses. Vaccine 30(Suppl 5):F55–F70. doi:10.1016/j.vaccine.2012.06.083PubMedCrossRef
Dorleans F, Giambi C, Dematte L, Cotter S, Stefanoff P, Mereckiene J, O Flanagan D, Lopalco P, D’Ancona F, Levy-Bruhl D (2010) The current state of introduction of human papillomavirus vaccination into national immunisation schedules in Europe: first results of the VENICE2 2010 survey. Euro Surveill 15(47)
Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Meric D, Dessy FJ, Datta SK, Descamps D, Dubin G, Group HPVS (2011) Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12–24 in a Phase III randomized study of healthy women aged 18–45 years. Hum Vaccin 7(12):1343–1358. doi:10.4161/hv.7.12.18281PubMedCentralPubMedCrossRef
Esposito S, Birlutiu V, Jarcuska P, Perino A, Man SC, Vladareanu R, Meric D, Dobbelaere K, Thomas F, Descamps D (2011) Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine administered according to an alternative dosing schedule compared with the standard dosing schedule in healthy women aged 15 to 25 years: results from a randomized study. Pediatr Infect Dis J 30(3):e49–e55. doi:10.1097/INF.0b013e318206c26ePubMedCrossRef
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917. doi:10.1002/ijc.25516PubMedCrossRef
Garland SM, Skinner SR, Brotherton JM (2011) Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 53(Suppl 1):S29–S35. doi:10.1016/j.ypmed.2011.08.015PubMedCrossRef
Garnett GP (2005) Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis 191(1):S97–S106PubMedCrossRef
Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, Kulldorff M, Lewis E, Fireman B, Daley MF, Klein NP, Weintraub ES (2011) Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine 29(46):8279–8284. doi:10.1016/j.vaccine.2011.08.106PubMedCrossRef
Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM (2013) Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med 11(1):227. doi:10.1186/1741-7015-11-227PubMedCentralPubMedCrossRef
Gillison ML, Alemany L, Snijders PJ, Chaturvedi A, Steinberg BM, Schwartz S, Castellsague X (2012) Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine 30(Suppl 5):F34–F54. doi:10.1016/j.vaccine.2012.05.070PubMedCrossRef
Giuliano AR, Lazcano-Ponce E, Villa L, Nolan T, Marchant C, Radley D, Golm G, McCarroll K, Yu J, Esser MT, Vuocolo SC, Barr E (2007) Impact of baseline covariates on the immunogenicity of a quadrivalent (types 6, 11, 16, and 18) human papillomavirus virus-like-particle vaccine. J Infect Dis 196(8):1153–1162PubMedCrossRef
Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, Chang YH, Ferris D, Rouleau D, Bryan J, Marshall JB, Vuocolo S, Barr E, Radley D, Haupt RM, Guris D (2011) Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med 364(5):401–411. doi:10.1056/NEJMoa0909537PubMedCentralPubMedCrossRef
Gold MS, McIntyre P (2010) Human papillomavirus vaccine safety in Australia: experience to date and issues for surveillance. Sex Health 7(3):320–324PubMedCrossRef
Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G (2006) Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367(9518):1247–1255PubMedCrossRef
Harris T, Williams DM, Fediurek J, Scott T, Deeks SL (2014) Adverse events following immunization in Ontario’s female school-based HPV program. Vaccine. doi:10.1016/j.vaccine.2014.01.004, doi:S0264-410X(14)00005-X [pii]
Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, Huh WK, Sings HL, James MK, Haupt RM, for the FI, Group IIS (2012) Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial. Bmj 344:e1401. doi:10.1136/bmj.e1401PubMedCentralPubMedCrossRef
Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, Robertson C, Donaghy M (2014) Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. doi:10.1038/bjc.2014.198, bjc2014198 [pii]
Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT (1992) Papillomavirus L1 major capsid protein self assembles into virus like particles that are highly immunogenic. In: Proceedings of the National Academy of Sciences of the United States of America 89, pp 12180–12184, 24 Dec 15
Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Lehtinen M, Steben M, Bosch FX, Dillner J, Joura EA, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Maansson R, Lu S, Vuocolo S, Hesley TM, Saah A, Barr E, Haupt RM (2009) A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2(10):868–878. doi:10.1158/1940-6207.CAPR-09-0031CrossRef
Labadie J (2011) Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 23(2):103–112. doi:10.3233/JRS-2011-0529, V571338816652H47 [pii]PubMed
Lacey CJ, Lowndes CM, Shah KV (2006) Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 24(Suppl 3):S3/35–41
Lamontagne DS, Thiem VD, Huong VM, Tang Y, Neuzil KM (2013) Immunogenicity of quadrivalent HPV vaccine among girls 11 to 13 Years of age vaccinated using alternative dosing schedules: results 29 to 32 months after third dose. J Infect Dis 208(8):1325–1334. doi:10.1093/infdis/jit363, jit363 [pii]PubMedCrossRef
Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, Chow SN, Kitchener H, Teixeira JC, Hedrick J, Limson G, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, De Carvalho NS, Germar MJ, Peters K, Mindel A, De Sutter P, Bosch FX, David MP, Descamps D, Struyf F, Dubin G (2012) Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13(1):89–99. doi:10.1016/s1470-2045(11)70286-8PubMedCrossRef
Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, Unger ER (2013) Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, national health and nutrition examination surveys, 2003–2010. J Infect Dis 208(3):385–393. doi:10.1093/infdis/jit192PubMedCrossRef
Mesher D, Soldan K, Howell-Jones R, Panwar K, Manyenga P, Jit M, Beddows S, Gill ON (2013) Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. doi:10.1016/j.vaccine.2013.10.085PubMedCentralPubMed
Moscicki AB, Wheeler CM, Romanowski B, Hedrick J, Gall S, Ferris D, Poncelet S, Zahaf T, Moris P, Geeraerts B, Descamps D, Schuind A (2012) Immune responses elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in previously vaccinated adult women. Vaccine 31(1):234–241. doi:10.1016/j.vaccine.2012.09.037PubMedCrossRef
Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia PJ, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Huh WK, Joura EA, Kurman RJ, Majewski S, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan JT, Lupinacci LC, Giacoletti KE, Sings HL, James MK, Hesley TM, Barr E, Haupt RM (2010) Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on All HPV-associated genital diseases in young women. J Natl Cancer Inst 102(5):325–339PubMedCrossRef
Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Hoye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, von Krogh G, Lehtinen M, Paavonen J, Tamms GM, Giacoletti K, Lupinacci L, Esser MT, Vuocolo SC, Saah AJ, Barr E (2007) Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 25(26):4931–4939PubMedCrossRef
Pagliusi SR, Teresa Aguado M (2004) Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 23(5):569–578, Dec 16PubMedCrossRef
Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, Marshall JB, Radley D, Vuocolo S, Haupt RM, Guris D, Garner EI (2011) HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 365(17):1576–1585. doi:10.1056/NEJMoa1010971PubMedCrossRef
Parkin DM, Bray F (2006) Chapter 2: the burden of HPV-related cancers. Vaccine 24(Suppl 3):S3/11–25
Read TR, Hocking JS, Chen MY, Donovan B, Bradshaw CS, Fairley CK (2011) The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect 87(7):544–547. doi:10.1136/sextrans-2011-050234PubMedCrossRef
Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, Puchalski D, Giacoletti KE, Sings HL, Lukac S, Alvarez FB, Barr E (2007) Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 26(3):201–209PubMedCrossRef
Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT (2007) Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 13(7):857–861PubMedCrossRef
Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Catteau G, Dobbelaere K, Schuind A, Descamps D (2011) Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin 7(12):1374–1386. doi:10.4161/hv.7.12.18322PubMedCentralPubMedCrossRef
Roteli-Martins C, Naud P, De Borba P, Teixeira J, De Carvalho N, Zahaf T, Sanchez N, Geeraerts B, Descamps D (2012a) Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother 8(3):390–397PubMedCrossRef
Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B, Descamps D (2012b) Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother 8(3):390–397. doi:10.4161/hv.18865, 18865 [pii]PubMedCrossRef
Rowhani-Rahbar A, Mao C, Hughes JP, Alvarez FB, Bryan JT, Hawes SE, Weiss NS, Koutsky LA (2009) Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 27(41):5612–5619PubMedCentralPubMedCrossRef
Rowhani-Rahbar A, Alvarez FB, Bryan JT, Hughes JP, Hawes SE, Weiss NS, Koutsky LA (2012) Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 53(3):239–243. doi:10.1016/j.jcv.2011.12.009PubMedCentralPubMedCrossRef
Schiller JT, Castellsague X, Garland SM (2012) A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 30(Suppl 5):F123–F138. doi:10.1016/j.vaccine.2012.04.108PubMedCrossRef
Shope RE (1937) Immunization of rabbits to infectious papillomatosis. J Exp Med 65:607–624CrossRef
Siegrist CA, Lewis EM, Eskola J, Evans SJ, Black SB (2007) Human papilloma virus immunization in adolescent and young adults: a cohort study to illustrate what events might be mistaken for adverse reactions. Pediatr Infect Dis J 26(11):979–984PubMedCrossRef
Silverberg MJ, Thorsen P, Lindeberg H, Grant LA, Shah KV (2003) Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet Gynecol 101(4):645–652PubMedCrossRef
Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, Izurieta HS, Ball R, Miller N, Braun MM, Markowitz LE, Iskander J (2009) Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 302(7):750–757PubMedCrossRef
Stanley M (2008) Immunobiology of HPV and HPV vaccines. Gynecol Oncol 109(2 Suppl):S15–S21PubMedCrossRef
Stanley M, Lowy DR, Frazer I (2006) Chapter 12: prophylactic HPV vaccines: underlying mechanisms. Vaccine 24(Suppl 3):S3/106–113
Suzich JA, Ghim SJ, Palmer Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R (1995) Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A 92(25):11553–11557, Dec 5PubMedCentralPubMedCrossRef
Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, Bateson D, McNamee K, Garefalakis M, Garland SM (2012) Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis 206(11):1645–1651. doi:10.1093/infdis/jis590PubMedCrossRef
Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, Brown DR, Ferenczy A, Harper DM, Koutsky LA, Kurman RJ, Lehtinen M, Malm C, Olsson SE, Ronnett BM, Skjeldestad FE, Steinwall M, Stoler MH, Wheeler CM, Taddeo FJ, Yu J, Lupinacci L, Railkar R, Marchese R, Esser MT, Bryan J, Jansen KU, Sings HL, Tamms GM, Saah AJ, Barr E (2006) Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 24(27–28):5571–5583, Jul 7PubMedCrossRef
Winer RL, Feng Q, Hughes JP, O’Reilly S, Kiviat NB, Koutsky LA (2008) Risk of female human papillomavirus acquisition associated with first male sex partner. J Infect Dis 197(2):279–282PubMedCentralPubMedCrossRef
Woodhall SC, Jit M, Cai C, Ramsey T, Zia S, Crouch S, Birks Y, Newton R, Edmunds WJ, Lacey CJ (2009) Cost of treatment and QALYs lost due to genital warts: data for the economic evaluation of HPV vaccines in the United Kingdom. Sex Transm Dis 36(8):515–521PubMedCrossRef
Zhou J, Sun XY, Stenzel DJ, Frazer IH (1991) Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion like particles. Virology 185(1):251–257PubMedCrossRef