Eric Legome and Ashika Jain
Accidental trauma is the leading cause of death in the United States in the 1- to 44-year-old age group and the fifth leading cause overall. In the United States, injuries cost an estimated $117 billion in medical expenditures each year. This represents approximately 10% of total medical spending, similar in magnitude to the medical costs associated with other leading public health concerns. These estimates do not include the lost productivity, nonmedical expenditures, insurance costs, property damage, litigation, decreased quality of life, and long-term noninjury health consequences (e.g., mental health care costs). In addition, the personal and social costs owing to premature loss of life or livelihood is immeasurable (1,2).
Optimal care of the trauma patient begins in the prehospital setting, continues throughout the emergency department (ED) course, and extends into the intensive care and rehabilitation phases. All of these parts must work in concert to provide for the best outcome. Although not always feasible, the outcome for the severely injured trauma patient is optimized when they are initially treated at, or expeditiously transferred to, a regional trauma center with expertise in and dedication to all facets of trauma care (3–5).
In addition to treatment of the individual patient, resources must be allocated to the prevention of trauma, an often underappreciated aspect of trauma care. Even minor changes in traffic safety, alcohol and drug use, and gun safety have a tremendous potential to decrease trauma morbidity (4,6).
CLINICAL PRESENTATION
Prehospital
After extrication and immobilization, rapid transport to a trauma center is the most efficacious prehospital trauma intervention. Many prehospital interventions such as military antishock trousers for hypotensive patients have not been found to be helpful, and even interventions such as rapid-sequence intubation (RSI) in the head-injured patient, once thought invaluable, have become controversial. The use of intravenous fluids has likewise become controversial, with timing, amount, and end point a current focus of contention. Current and future studies may offer a clearer understanding of which prehospital interventions have the most utility (5,7).
There are several reasons for rapid transport to a trauma center. While community hospitals have physicians with excellent skills and training in managing trauma patients, major trauma centers evaluate a significantly higher number of major trauma patients with appropriate resource allocation. Furthermore, their personnel are more likely to have advanced skills, appropriate ancillary staffing, up-to-date technology, readily available consultants, rapid operating room (OR) capability, intensive care unit (ICU) capability, and rehabilitative care to restore a patient to full health. In addition, when compared to nontrauma centers, they have been shown to decrease death rates in the most seriously injured patients (3,5). Some areas do not have an available trauma center; for example, there are no major level 1 trauma centers in rural states such as Idaho or Montana (8). In these areas, it may be prudent to take a high-risk patient to a center with available surgical and interventional capability, even if it means bypassing a closer hospital. Often a local hospital will have to stabilize a patient prior to transfer to a convenient trauma center. Therefore, transfer agreements, routes and the mechanics of evacuation should be worked out well in advance of the individual patient’s need. These decisions are often community-specific and based on an understanding of local resources, demographics, and transport times. Regardless of the setting, most emergency physicians (EPs) will encounter both major and minor trauma throughout their career, and competence and confidence in caring for these patients is essential to the skill set of the EP.
Due to funding constraints, a number of hospitals have closed their trauma service because of an inability to support the expensive personnel and equipment needs of a 24-hour service. Moreover, lack of reimbursement, along with the risk of malpractice suits that often accompany major trauma, has discouraged many specialists from participating in trauma care. Subsequently, this has led to a crisis in the ability to deliver timely and appropriate subspecialty care (9).
Emergency Department
On arrival to the ED, trauma patients with abnormal physiologic parameters, a major mechanism of injury or a potentially serious injury should be triaged to a suitable treatment area capable of major resuscitation. It is safer to initially overestimate the degree of injury and then downgrade the patient after initial evaluation, rather than upgrade a patient to more aggressive care. Appropriate personnel should accompany the patient to the resuscitation room to ensure that adequate life-support measures are being accomplished en route. Details regarding the mechanism of injury may offer important clues in predicting potential injuries.
Mechanism of injury must always be taken seriously and is essential for injury evaluation. While a significant mechanism may not lead to major injury, and a trivial mechanism may cause severe morbidity, knowing the mechanism should heighten the suspicion for significant trauma. The most sensitive way to prognosticate major injury is to take both physiologic parameters and mechanism into consideration (Table 18.1).
TABLE 18.1
Mechanism of injury

Triage must take into account the immediate threat to life and limb and patient discomfort and anxiety. A variety of options exist for triage including emergency nurses, a dedicated trauma nurse and even a triage physician.
Major trauma is best evaluated by a dedicated trauma team. The team must have a captain, and the training and experience of this person may vary and should be appropriate to the specific hospital. Usually, the captain is initially an EP; given the increasing economic pressures and the decrease in number of trained trauma surgeons, there is increasing movement to involve trauma surgeons only after full ED evaluation or if urgent operation is required. EPs have also undergone advanced training in trauma care and may have joint appointments with surgical departments. This has led to ongoing controversy, and there is no widespread agreement on when or whether the trauma surgeon is needed routinely in the initial resuscitation (10–12). In some settings, an EP may be the only physician physically present in the ED (and, for that matter, in the hospital). Ideally, surgical support personnel are available within 10 to 30 minutes.
In trauma centers, surgical support is generally rapidly available within the hospital. In this system, the EP may assume the role of trauma captain for the initial assessment and resuscitation. When the attending trauma surgeon arrives, a defined transition of leadership may occur. The team concept must be maintained at all times.
If the responsibility for the major orchestration is formally handed over to the attending trauma surgeon, it does not relieve the EP of responsibility for the patient. The EP should assist until the patient physically leaves the ED for the OR, ICU, or special diagnostic study. Exceptions of course are made if there are other critical patients who demand the attention of the EP. When the EP has to leave the patient, the departure should be coordinated with the team and the team leader. The team may summon subspecialty consultations, but there must be a single person, generally the EP or trauma surgeon, in charge of the orchestration of total patient needs.
Nurses who care for trauma patients should have the requisite special knowledge and skills. In large urban centers, there is usually an abundance of physicians to perform resuscitative procedures, but in the rural ED, the trauma nurse may require technical skills ordinarily performed by physicians. Other institutions may use a combination of midlevel providers including specially trained nurse practitioners or physician assistants. Prehospital personnel who do not need to immediately return to the field may be requested to remain in the ED so that their technical skills can be utilized.
The area of the radiology department designated for emergency patients should optimally be adjacent to the ED. As radiology has become essential to trauma management, the radiologist must be considered a member of the trauma team and should be readily available for consultation. The ability to perform portable radiographs in the trauma resuscitation room is necessary since these patients should not be moved from the area during the initial stages of evaluation. Likewise point of care ultrasound (US) has become common in trauma centers to provide immediate information at the bedside. Protocols and guidelines should be available regarding expectations of all ancillary staff. A coordinated, knowledgeable response from the blood bank, laboratory, respiratory therapy, and radiology department is vital to an effective trauma system. Social workers, clergy, and lay support should be included in the team framework.
The OR must be rapidly accessible to the ED for operative resuscitation of critically injured patients. Some centers have emphasized this by having an OR as part of the ED or by reserving a single OR for trauma use, but others have utilized a special OR in the surgical suite in which to perform the initial resuscitation.
ED EVALUATION AND MANAGEMENT
Prior to Arrival
When notice is given by EMS that a critical patient is en route, the ED should consider potential needs, for example, having blood for an exsanguinating patient. The entire trauma team should ensure standard precautions, with gloves, gowns, masks, and protective eyewear. All patients should be presumed to be potentially infectious from blood-borne or body fluid–borne diseases and should be treated accordingly until risk can be fully assessed. These precautions apply to blood and to other body fluids containing visible blood, synovial fluid, pleural fluid, peritoneal fluid, pericardial fluid, and amniotic fluid. Prior to arrival, the team captain should assemble the team and assign roles; this may include setting up for necessary procedures (i.e., airway management and tube thoracostomy).
Primary Survey
The approach to the trauma patient begins with a rapid initial examination. While the initial assessment proceeds in a stepwise approach, when performed with multiple providers, several steps may occur simultaneously. This is appropriate as long as one clinician is clearly overseeing the entire process. On arrival, the patient must be immediately undressed. Care should be taken to avoid hypothermia by placing warm blankets on the patient after the evaluation is done. The cervical spine should be immobilized until it is cleared of injury by radiographic or clinical criteria, but early relief from the back board is critical to patient comfort.
The initial trauma assessment, or the ABCs, begins with evaluation of the airway. The need for airway management is the most critical initial question in the patient with multiple trauma. Table 18.2 lists common criteria that serve as guidelines for this decision.
TABLE 18.2
Indications for Intubation
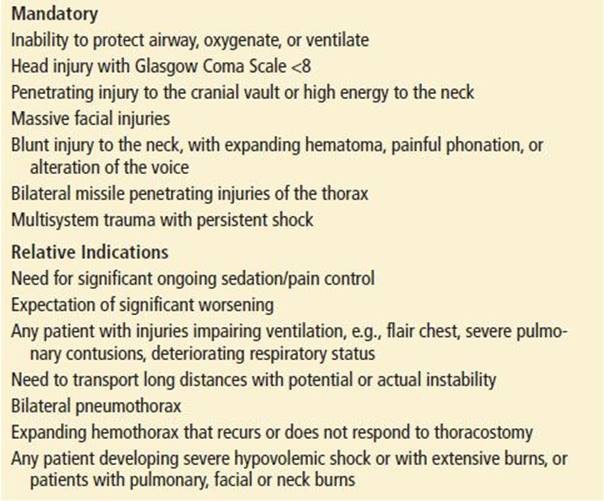
The airway assessment should include patency, ability to speak clearly, and ability to control secretions. If this is not the case, the EP should search for possible obstruction in the form of foreign body or relaxed tongue that might obstruct the airway. Oxygenation of the tissues is critical, and clearing the airway is the initial step in oxygen delivery. A suction apparatus may be used to clear the upper airway. The patient with facial injuries, but no neck injuries, may be turned onto the side or sat up leaning forward to allow rapid clearing of the airway. A patient may also be turned on the backboard or while maintaining spinal immobilization (log rolling) if the condition of the cervical spine is unknown.
An oral airway may be placed in the unconscious trauma victim to allow for oxygenation but this is generally followed rapidly by intubation. In some cases of severe maxillofacial trauma or direct tracheal injury, cricothyrotomy is indicated. The need for intubation and active airway management is a clinical decision and should never be withheld pending a blood gas. Once the airway is patent, breath sounds should be rechecked.
If the patient is not breathing spontaneously or adequately, ventilation must be controlled. Initial control may be by bag-valve-mask ventilation with high-flow supplemental oxygen. Once the patient is oxygenated and hypercapnia has been corrected, intubation is less hazardous, but undertaking bag-valve-mask ventilation must be weighed against the risk of distending an already full stomach.
Major trauma victims often need early airway management, even if they are breathing spontaneously. Patients may have injuries distorting their airways (e.g., a gunshot wound to the neck). It is prudent to intubate these patients early in their course before intubation is impossible to perform such as if the neck is distorted by a large hematoma. In addition to airway protection and maintenance of anatomic function, other reasons to intubate the major trauma patient exist. Intubation will allow for multiple tests and transport in patients with serious injuries or altered mental status who may require ongoing sedation or narcotic pain control. In some cases, airway management is indicated owing to the expectation that the injuries are so severe that the patient will eventually need intubation. Patients with severe or suspected head injury with a Glasgow Coma Score (GCS) of <8 or a declining mental status should be intubated to protect the airway, prevent hypoxia, and allow for controlled breathing. Finally, because few trauma patients have an empty stomach at the time of injury, intubation can help lower the risk of vomiting and aspiration.
Oral intubation using RSI is the optimal approach in the ED. It has been very successful and has virtually eliminated the need for cricothyrotomy. The preferred approach to intubation in the patient with a possible cervical spine injury is to keep the neck immobilized by inline stabilization and perform RSI. A second pair of hands other than those of the intubator provides in-line stabilization of the head and neck, which also allows for opening the anterior collar.
Nasal intubation, which is still occasionally performed in some prehospital systems, is rarely used in the ED. Many EDs are commonly using specialized approaches to the airway, especially video-assisted laryngoscopy, often as the initial procedure (13). A surgical airway may be necessary if there is extensive damage to the patient’s larynx, complete tracheal separation, or significant midfacial or orofacial trauma or neck distortion.
Laryngeal mask airway may be used as a rescue technique in the ED but generally does not provide a long-term secure and safe airway and should be replaced as soon as possible by a secure endotracheal tube.
After evaluation and management of the airway, breathing and circulation must be assessed.
In assessing breathing, the physician should observe the chest for sucking wounds or paradoxical motion, palpate for bony crepitus or subcutaneous air, and auscultate for breath sounds. Severe dyspnea, hypoxia, subcutaneous air, or tachypnea in the presence of penetrating chest trauma should lead to the presumption of an underlying hemo- or pneumothorax.
The major causes of pulmonary dysfunction in the trauma patient are hemo- and pneumothorax. The patient may also hypoventilate owing to multiple rib fractures or underlying pulmonary contusions.
Tension pneumothorax causes shock by increasing intrathoracic pressure, shifting the mediastinum, and compressing the great veins, thereby preventing venous return. The diagnosis is probable in a trauma patient in shock who has distended neck veins and an increased resistance to bagging. An ipsilateral decrease in breath sounds may be noted and a contralateral tracheal shift. Unfortunately, these physical findings are often not present acutely and the only clues to the presence of this may be a sudden rise in pulse, a drop in blood pressure, and an increased resistance to ventilation. If tension pneumothorax is suspected in the field, it should be immediately decompressed with a large-bore over-the-needle catheter in the fifth intercostal space or the second intercostal space in the midclavicular line. Treatment of a suspected tension pneumothorax should not be delayed to obtain radiographic confirmation. The physician should needle decompress the chest or perform thoracostomy by inserting a large-bore chest tube in the fifth intercostal space, midaxillary line, to evacuate any blood or air.
After pulmonary evaluation, circulation must be rapidly assessed. Any obviously bleeding source should be controlled by pressure, and vascular access should be obtained. There is no single answer to which method of intravenous access is the best. Each patient must be evaluated individually, and the risk–benefit ratio of the chosen routes weighed. In the patient in shock or with concerning findings, it is best to insert a minimum of two large-bore (14 to 16 gauge) peripheral lines, adding more as necessary. Patients with minimal or no injuries may not require such aggressive intervention. Sometimes it may be necessary to proceed immediately to a central line or peripheral cut down to gain access. A central venous catheter may be helpful in monitoring central volume, in addition to delivering fluid and blood. Central line placement utilizing an US probe has become a much safer procedure and will not only give access with a large-bore catheter but allow successful central venous pressure monitoring. Intraosseous techniques, more common in children, may be used in adults also. This has become more common, especially in the prehospital arena when standard access is unobtainable (14).
Most traumatized adults can tolerate generous fluid infusions because they are usually young and previously in good health. Whether this is best however, is still unclear. Classic teaching has been that an initial dose of 20 mL/kg is safe and an appropriate starting point. If the patient still shows signs of significant volume deficit after administration of 50 mL/kg or 2 L of crystalloid, type-specific or O-negative blood should be started. In the patient who has an injury that obviously will produce a major volume deficit (e.g., shotgun blast with marked tissue destruction and loss), blood should be given very early. Massive transfusion protocols have become much more common, based largely on experiences in the military. While there is little prospective research showing their benefit, retrospective case control studies suggest that when performed with appropriate ratios of blood to platelets and factors, they may be effective in decreasing mortality. When massive transfusions are used, they generally should be part of a hospital wide strategy and protocol (15–17).
Both the end point and the aggressive use of fluid resuscitation are controversial. Owing to concerns, based mostly on animal studies, that aggressive fluid resuscitation to normotension in the actively bleeding patient may lead to increased mortality, a less assertive response to hypovolemia has been advocated. This mortality is due to factors such as increased bleeding from increased arterial and venous pressure, dilution of clotting factors, clot disruption, and decrease in blood viscosity. Multiple animal studies have upheld this idea of “hypotensive resuscitation.”
In clinical practice, however, it has yet to be fully proven beneficial. Few significant studies have been performed in human beings, one with penetrating and one with a combination of penetrating and blunt trauma patients. Although one study found a benefit to not actively resuscitating patients with penetrating torso trauma, there have been concerns about the methodology and validity of the study. In addition, patients with severe head injury have a worsened outcome with hypotension, making this approach unlikely to be helpful in this group of patients. However, many clinicians and academic centers believe strongly in the concept of less aggressive resuscitation. This approach should be considered as an appropriate alternative while awaiting more definitive research in this area (18–20).
The amount of fluids administered depends on continued losses, which should be minimized as much as possible. Though intra-abdominal bleeding may require definitive surgical or angiographic intervention, interventions in the ED such as proper splinting, bandaging, and avoidance of probing large soft tissue wounds will maximize hemorrhage control. The degree to which long-bone and pelvic fractures bleed is not generally appreciated; it is far more effective to administer blood when these injuries are recognized than to wait until the patient is in shock. Direct pressure should be applied to control external hemorrhage. Blind probing and clamping deep within a wound may cause further injury to vessels and nerves. A soft tissue injury, especially to the scalp, may result in large amounts of bleeding. The best treatment for hypovolemia is to locate the source of bleeding and stop the blood loss. Hemostasis can be rapidly obtained in a scalp laceration either by the use of Raney clips (neurosurgical skin clips that can be quickly applied to the scalp laceration edges) or by placement of a 2-0 nylon running locked stitch.
While most trauma patients in shock are hypovolemic and this should be actively pursued, other options should still be considered. Cardiac causes of shock must also be in the differential diagnosis. Pericardial tamponade is rare with survivable closed-chest injuries; another source of the shock should probably be sought. It must always be considered with penetrating injuries, even if the entrance wound appears anatomically remote. The physiologic response to tamponade is to compensate for the fall in cardiac output by raising the pulse rate. The classic physical findings may be absent in the acute situation. Thus, one may not see distended neck veins if the patient is profoundly hypovolemic. Furthermore, heart sounds may be difficult to appreciate in the ED. The use of focused assessment with sonography for trauma (FAST) in the trauma bay is highly useful in evaluating for pericardial fluid. If not available in the trauma bay a pericardiocentesis can be performed. If, on the other hand, the patient has just lost vital signs an immediate thoracotomy should be performed.
Blunt cardiac injury may cause dysrhythmias. An electrocardiogram should be obtained in patients with significant anterior blunt chest trauma. Continuous cardiac monitoring is indicated. A normal EKG and period of monitoring in the ED generally rules out a significant blunt cardiac injury. Myocardial infarction may occur as a result of the stress associated with blood loss and catecholamine release, or it may have preceded the traumatic injury.
The presence of spinal cord trauma with loss of sympathetic vascular tone can be assessed by rectal examination and examination of peripheral reflexes. The extremities should be palpated; if they are cool, moist, or pale, hemorrhagic shock must be presumed.
If the patient has a cardiac arrest from hypovolemia or pericardial tamponade, immediate emergency thoracotomy may be indicated. In general, thoracotomy in the presence of penetrating injury and a patient who arrests in the ED or just prior to arrival offers the best prognosis. Maximal benefits are obtained in the presence of low-velocity penetrating injury to the chest, particularly the heart. Blunt trauma arrests in general have an extremely poor prognosis, especially with a prehospital arrest or multiple concomitant injuries (9).
Operative intervention is part of the resuscitation of the unstable trauma victim. In the patient unable to be resuscitated from shock without an obvious source of blood loss, immediate exploration may be indicated. A chest radiograph helps determine whether the chest or the abdomen is the site of initial exploration.
Patients with penetrating trauma, in the absence of contraindications, should be rolled onto the side to ensure that there are no wounds on the back, buttocks, or in the axillae. Blood pressure determination from the field should be repeated. Although not clearly correlated with a specific blood pressure, locating a palpable pulse provides information about the perfusion pressure.
When intravenous lines are placed, blood is drawn for typing and other tests. If possible, the prehospital providers may draw blood. Though it may be more efficient to use a large, 50-mL syringe and simultaneously draw all needed blood samples and measure arterial blood gas from the femoral artery, this may also increase the possibility of needle sticks when the blood is transferred. It is the trauma captain’s responsibility to ensure that blood is obtained in a timely manner.
The priorities of definitive management are still vigorously debated, and it is often necessary to perform invasive procedures without the luxury of confirmatory diagnostic studies. In general, one should attempt to approach the pathology that poses the most immediate threat to the patient’s life—usually in the abdomen.
Secondary Survey
In the patient who stabilizes during resuscitation, the next priority is a complete head-to-toe examination, including repeat maxillofacial, cardiopulmonary, abdominal, neurologic, and orthopedic examinations. In addition to the examination, further history about the timing and mechanism of the injury should be obtained with the past medical history, medications, and allergies.
If the patient is unconscious, determining the level of brainstem function and the presence of a herniation syndrome is paramount. Most trauma victims die of central nervous system–related injuries. While in the stable patient with little clinical concern the rectal examination is rarely helpful and may be misleading, in the multiple trauma patient, a rectal examination may reveal displacement of the prostate, or gross blood, and provide for neurologic assessment of the anal sphincter tone and sensation (21). The absence of tone or sensation may indicate a spinal cord injury in a patient previously assessed to have only a head injury.
It is necessary to immobilize the spinal column until its status can be ascertained, but it is not necessary to spend long periods of time trying to clear the cervical or other spinal column before dealing with more life-threatening injuries. The patient can be maintained supine with a hard collar while more immediate life threats are determined.
Open fractures should be gently irrigated (realizing that this procedure will not substitute for more effective and complete irrigation and debridement under anesthesia) and splinted to prevent further injury and reduce pain and bleeding. Open fractures should be covered with clean dressings and intravenous antibiotics administered, to provide antistaphylococcal coverage. Definitive care may have to be delayed while surgical treatment of more serious, life-threatening injuries occurs.
Laboratory and Diagnostic Studies
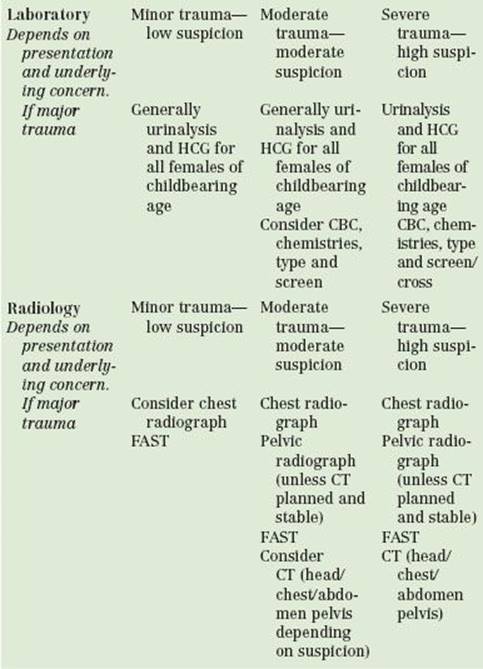
Laboratory analysis of the trauma patient is generally more extensive than necessary. Routine laboratory studies on preoperative patients are not useful or cost effective unless indicated by the history and physical. However, owing to the limitations in obtaining this information in the multiple trauma patient, an extensive array of tests is often completed. Though testing based on clinical parameters has been shown to be cost effective without compromising care, most trauma centers use some type of protocol to standardize and speed the laboratory evaluation of patients (22). The patient who is fully awake and alert, who has sustained minimal trauma, and who has no distracting injuries may require no testing. Rational laboratory analysis of most patients with moderate trauma can probably be limited to a complete blood count, a basic metabolic panel, blood type and screen, and urinalysis.
Clinical screening for basic risk factors for renal insufficiency (i.e., history of renal disease or diabetes) in ED patients has been repeatedly shown to be an excellent screening tool for patients who need a serum creatinine prior to receiving intravenous contrast for CT. If risk factors are absent, an urgent CT scan should not be withheld while awaiting serum creatinine. The multiple trauma patient or one about to undergo urgent operative management may require more thorough testing. Women of childbearing age should have a pregnancy test performed.
The sequencing of radiologic studies should be prioritized in the same manner as the other components of the resuscitation. Though cervical spine imaging is essential in the patient who cannot be clinically cleared, the imaging may be delayed and the patient kept in a collar and immobilized while addressing more pressing issues. In victims of blunt trauma, chest and pelvic films should be obtained next and, if necessary, extremity films obtained thereafter. The chest radiograph should be taken as soon as possible in the patient with penetrating injury, although with proper training, the test characteristics of bedside US for lung pathology are excellent and arguably better than radiographs (23). Empiric treatment of a presumed pneumo- or hemothorax should take priority if the patient is unstable. Clinical evaluation of the pelvis without radiographic study is accurate to exclude a clinically important fracture. It is still wise to routinely obtain a study on the patient with altered mental status, hypotension or significant distracting injury and appropriate mechanism (24).
One of the most important decisions in the management of the multiple trauma patient is when and how to obtain an objective evaluation of the abdomen. Generally a combination of mechanism, physical findings and age are the most important factors. With a major mechanism of injury even in the well-appearing patient, it is reasonable to consider further evaluation of the abdomen for occult hemorrhage. Clinical judgment, however, should be used (25). The physical examination of the abdomen is less reliable when there are multiple injuries, competing pain from skeletal trauma, or altered mental status from head injury, shock, or substance abuse. In the presence of these factors, objective evaluation should generally be obtained. Finally, in any patient who demonstrates hemodynamic instability without obvious source, it is critical to evaluate the abdomen for hemorrhage.
Objective evaluation of the abdomen is generally performed by one of two common methods: CT or US. While previously a common option, diagnostic peritoneal lavage (DPL) has largely been replaced by US (26).
DPL is highly accurate (92% to 98%); it has reliable significance when negative. However, as noted, it has largely been supplanted by CT and US, both noninvasive tests. DPL is still recommended in the unstable patient with an unclear US result or in the stable patient with a significant concern for a hollow viscous or mesenteric injury, either by examination or after an equivocal CT. The only absolute contraindication for DPL is the need for emergency laparotomy.
Ultrasonography can also be performed at the bedside. The FAST examination, a four-view, directed US examination, is able to reliably find fluid within the abdomen and within the pericardial sac. It is accurate with significant intra-abdominal hemorrhage, and as it is noninvasive, it has mostly replaced the DPL. FAST is performed on the supine patient with the examiner positioned on the right side. Four locations are scanned: (a) the right upper quadrant, (b) the left upper quadrant, (c) the suprapubic region, and (d) the pericardium. The examiner looks for unclotted blood, a black or anechoic finding on the US’s screen. It is reasonably reliable, especially with enough bleeding to cause hemodynamic instability, but may need to be performed more than once if the patient is seen very soon after the trauma prior to large amounts of bleeding. The level of accuracy is comparable regardless of whether an EP, surgeon or radiologist performs the study. The major limitation of US is that it shows fluid in the abdomen but not its source or the magnitude of injury. Therefore, a positive US in a stable patient is generally followed by a CT scan. An advantage of US is that the heart can be assessed for pericardial effusion. The bedside US has good diagnostic accuracy for long-bone fractures and hemothorax and is more accurate than supine chest radiograph for detection of a pneumothorax (23).
Though the main use for US is in blunt abdominal trauma, a positive FAST in a penetrating trauma patient has an extremely high positive predictive value for an operative lesion. A negative test cannot rule one out (27).
CT offers both quantitative and qualitative information. It is the gold standard for evaluation of the abdomen in the hemodynamically stable blunt trauma patient. It may also be used to evaluate penetrating trauma, although with greater limitations. With the advent of the multidetector CT, the test is performed rapidly with excellent accuracy. These scanners also allow for reformations of areas of concern for pathology, including the spine and aorta. The major limitation of abdominal CT is the decreased sensitivity for bowel, mesenteric, diaphragmatic, and pancreatic injury, although there is usually some direct or indirect sign of injury such as unexplained free fluid (28,29). A normal CT in trauma has an extremely high negative predictive value for operative intra-abdominal pathology and should provide clinicians with a very high probability that they are not missing an operative lesion (30). Of course, technology and those who interpret its data are not infallible. If the clinical examination still suggests the possibility of injuries not revealed on CT, the patient should undergo further testing or observation.
Additional evaluation depends on specific concerns. Gross hematuria should prompt evaluation of the genitourinary (GU) tract. Microscopic hematuria is poorly sensitive and specific for significant GU injury and, in the absence of a pelvic fracture, gunshot wound of the abdomen, or unexplained hypotension after blunt trauma, does not mandate workup. Penetrating trauma with any hematuria should be followed by a formal evaluation of the GU tract. Gross penile bleeding should be evaluated by retrograde urethrography before passage of a Foley catheter.
Many modern EDs have a scanner either in or immediately adjacent to the ED. The speed of the newest scanners along with their proximity to the ED has allowed for safer use in patients who previously would not have been scanned owing to borderline blood pressure or concern over absolute stability. This must be done with extreme caution, however, as resuscitation is best not performed in CT. One approach that some trauma centers are now using is to perform a CT from head to pelvis, that is, the pan-scan. This may be cost effective as the extra time is measured in minutes, there is no increased use of film if the images are stored on a picture archival retrieval system, and scans of the bones can be included, obviating the need for plain films. Reformations allow for the ability to image the aorta and have essentially replaced aortography in the diagnosis of aortic injury. Radiation exposure, though not acutely dangerous, is essential to consider when making decisions about performing CT scans, especially in young patients. Although the lifetime risk of developing cancer from CT scanning remains low it is still a real concern. Therefore, a CT should not be performed as a replacement for physical examination when the patient is fully evaluable (31–33).
Interventional angiography is increasingly important in the management of trauma patients. Angiography is the definitive modality in controlling hemorrhage from major pelvic fractures. In patients with ongoing hemorrhage from major pelvic trauma, angiography with embolization has been shown to be more effective and definitive than external fixation devices or operative packing. For stable patients with intraperitoneal injuries, if an actively bleeding spleen, liver, or kidney is visualized, a dedicated angiography team may be able to embolize the lesion and avoid laparotomy. Though effective, angiography still remains resource-intensive and is not readily available at all centers (34,35).
Angiography may also be used to diagnose arterial injuries in the neck or extremities. Doppler US may reveal flow impairment in the carotid or vertebral vessels after a whiplash trauma to the neck. Arteriography is then used to define the injury. Currently, many centers have switched to CT angiography as the definitive test for vascular injury and are using catheter-based angiography only for treatment or in equivocal cases. Other centers utilize magnetic resonance angiography in the stable patient. Another place for angiography is in the patient with a penetrating wound near a large vascular structure with massive tissue damage or evidence of distal pulse deficits. If the patient is unstable, however, it is more prudent to take the patient to the OR, obtain vascular control, and perform the angiography intraoperatively. In some cases, for example, possible aortic rupture, transesophageal echocardiography is an excellent option to perform in the OR in the patient unable to tolerate CT angiography.
Though the management priorities in patients with head, chest, and abdominal injuries still present a challenge, the critical ordering should be to diagnose and treat injuries to the abdomen, head, and then chest (aorta), in that order. However, the specific patient and clinical judgment may alter this approach. In addition, the rapid speed of the multidetector CT allows for the full body scan with little additional time once the patient is on the CT gurney. The initial priority is to assess for intraperitoneal bleeding. An US study or DPL that shows blood in the abdomen does not reveal the specific organ injured or the amount of damage, and the patient may also have evidence of intracranial injury. A head CT requires transfer to the radiology suite, but the rapidity of the procedure may enable a quick assessment of the head en route to the OR for laparotomy. It also adds little time to the assessment to take cuts of the chest and abdomen. If intraperitoneal bleeding is present in the unstable patient, then management of pelvic fractures or aortic injury must be delayed until after laparotomy.
Finally, it is imperative to recognize that the trauma patient needs prompt definitive care. Time wasted in the field, the ED, or the radiology suite may increase mortality. Sometimes, the trauma surgeon must proceed to the OR with a paucity of diagnostic information rather than have the patient continue to hemorrhage.
KEY TESTING
DISPOSITION
The stable trauma patient may spend a number of hours between the ED and the radiology department during the completion of diagnostic tests. During this time, the patient must be monitored to ensure that deterioration does not occur or that a quick transfer to the OR has not become necessary. This may be visual or with cardiac monitoring, depending on the clinical situation.
The patient remains the responsibility of the ED until admitted. Once admitted, overall ED care of the patient may depend on institutional policies and resources. However, the EP should assist in care of any patient who remains in the ED. Even if the trauma team monitors the patient in the radiology department, when the patient returns to the ED, it again becomes the EP’s responsibility to remain involved in the care of the patient, unless pre-existing protocols are in place and the trauma service has the proper manpower to observe the patient. After careful evaluation and a period of observation, many patients can be discharged from the ED.
Transfer of the patient to another institution depends on multiple factors. If the patient is unstable, the only rationale for transfer is to achieve a level of care higher than what is available at the original treating facility. It may be necessary to care for life-threatening injury (e.g., removal or salvage of a ruptured spleen) and then transfer the patient for more sophisticated neurosurgical, orthopedic, or thoracic management.
TRAUMA SCORING SYSTEMS
Trauma scores are an imperfect but commonly used tool in trauma systems. Prehospital providers, EPs, and inpatient trauma services use these scores for planning purposes and as a quality assurance screen to monitor system performance. Regional trauma planners use these scoring systems to compare institutions and assess resource needs and as a tool for performing research on the effects of different interventions.
Economic and geographic constraints have dictated that many trauma patients cannot be cared for in a trauma center. Unfortunately, it is extremely difficult to devise a system based on inpatient data that is accurate in the prehospital setting for triaging patients. Prehospital triage protocols are complicated by factors such as drugs, alcohol, and competing injuries, and many serious injuries are often hidden until there is a catastrophic decompensation. For example, most of the trauma scores utilize the GCS as part of their scoring estimation. Although the GCS is often applied in the field, it was not intended to be used until after all alcohol has been metabolized. As at least half of all major traumas involve patients who are intoxicated, the GCS is not likely to solely represent brain injury. It is also difficult to calculate the GCS in the intubated and paralyzed patient. Even mechanism of injury, which provides useful clues to the seriousness of the forces involved, is inaccurate because the recipients of those forces sustain such variable degrees of injury. Data suggest that physiologic criteria and altered mental status are the best predictors of the need for major interventions; however, these criteria are bound to miss serious occult injuries that may not present until later in the ED or hospital course. Even at the trauma center, the initial recognition of injury severity is often difficult, and the variations of response to what appears to be minor trauma can lead to increased morbidity or mortality.
The optimal scoring system should be easy to apply to patients and have validity and good interrater reliability. It must have easily measured and understandable criteria and should not be so complex that it is difficult to calculate or understand the outcome measurements. Most scoring systems are based on anatomic scoring systems, physiologic scoring systems or a combination of both (36).
In the prehospital system, scores may be used to monitor quality assurance and to assist in deciding whether a patient requires a major trauma center. They may also aid trauma centers in formulating their needs and levels of response prior to patient arrival. In the ED, scores may be chosen to assess the need for a trauma team response, the need for prophylactic intubation, the likelihood of occult injury, or the need for ICU admission.
Physiologic Scores
Physiologic scores are employed in prehospital decision making, to evaluate the appropriateness and efficacy of injury treatment and to prognosticate outcome.
Revised Trauma Score
The original trauma score evaluated respiratory rate, respiratory expansion, systolic blood pressure, capillary refill, and GCS. Owing to variability in assessing several of the components, a revised score was developed in 1989, using data from the Washington Hospital Trauma Center and the Major Trauma Outcome Study. The Revised Trauma Score (RTS) was broken into three components: GCS, systolic blood pressure, and respiratory rate. Numbers were assigned to each parameter, with a higher number representing improved function. As this score is based entirely on physiologic variables, there is a risk of inappropriate decision making when patients are scored early after injury. Though the RTS is a commonly used score, several factors contribute to decreasing its specificity. This score is partially driven by the GCS score. As mentioned earlier, the GCS is skewed by alcohol and drugs, but it is also skewed by how quickly the patient is evaluated after the head trauma. For example, many patients who are ultimately deemed minor head injuries would actually have a low GCS if examined immediately after being struck on the head.
Anatomic Scores
Injury Severity Score
The Injury Severity Score (ISS) was developed as an extension of the Abbreviated Injury Scale (AIS) (4), one of the original scoring systems. The AIS grade was developed by dividing the body into six regions (the thorax, abdomen, and visceral pelvis; head and neck; face; bony pelvis and extremities; and external structures) and utilizing the site with the worst injury from each region when calculating the overall score. The original ISS was devised by summing the squares of the highest AIS grade in each of the three most severely injured areas. This modified scale was shown to correlate with fatality from trauma. Death from trauma begins to rise significantly when the ISS is above 15. Unfortunately, both the AIS and the ISS can be calculated accurately only after the full extent of the patient’s injuries is known. The ISS is now commonly used as a quality assurance tool, but it has no role in decision making either in the ED or in the prehospital setting.
Other Scoring Systems
Trauma and Injury Severity Score
Though the ISS accounts for patients’ injuries, prediction of mortality should take into account factors that affect likelihood of survival. The trauma and injury severity score (TRISS) methodology incorporates the patient’s age and ISS into a score that predicts the chance of survival. Patients with outcomes different from those predicted by the formula may be candidates for quality assurance review. TRISSs are heavily weighted toward head injury. The W statistics, derived by comparison of a hospital’s results to those from the Major Outcome Trauma Study, portray the number of excess survivors per 100 patients (8).
A Severity Characterization of Trauma System
A severity characterization of trauma system (ASCOT) was developed based on analysis of the shortcomings of TRISS seen in the Major Trauma Outcome Study. In addition to the RTS variables, ASCOT adds scoring assignments for injury to three body regions (brain–spinal cord, thorax–neck, and abdomen-major vessel). In comparison, ASCOT seems to perform better than TRISS for adults with blunt trauma. Both TRISS and ASCOT appear adequate for pediatric patients.
Clinical Judgment
After reviewing the available scores and methodologies for triaging trauma patients, the practicing physician may wish to consider the role of clinical judgment. In a large study evaluating the ability of urban EMTs to assess trauma, none of the scores were found to perform better than EMT judgment. The physician considering the use of these scores will need to consider the setting in which the scores will be used. Most urban EMTs have the ability to recognize major trauma patients (37). Depending on the purpose for which they are being used, EDs have a number of scoring systems available for their use. The RTS may be used to monitor changes in a patient’s condition during the course of the patient’s initial resuscitation. Clearly, the use of mechanism of injury to activate the trauma team will lead to an overuse of that resource, but a degree of overtriage is accepted in the effort to include all potentially injured trauma patients. EMS systems commonly use the Prehospital Index or the RTS.
Common Pitfalls
• Failure to appreciate the severity of the mechanism of injury.
• Ascribing positive physical findings to a benign cause.
• Failure to anticipate patient needs prior to arrival.
• Failure to have a visible trauma captain throughout the resuscitation.
• Failure to use a well-thought-out, systematic approach to the patient.
• Chaotic and inefficient resuscitation because of poor use of team members or because too many physicians are trying to give orders.
• Focusing on subspecialty needs at the expense of real or potential life threats.
• Overemphasizing diagnostic tests in an unstable patient.
ACKNOWLEDGMENT
Authors Gratefully acknowledges the contribution of Peter Rosen to the content of this chapter.
REFERENCES
1. CDC. CDC health data interactive. CDC.gov. 2010.
2. CDC. http://www.cdc.gov/injury/wisqars/pdf/10LCID_All_Deaths_By_Age_Group_2010-a.pdf. 2010.
3. MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354(4):366–378.
4. Stewart RM, Myers JG, Dent DL, et al. Seven hundred fifty-three consecutive deaths in a level I trauma center: The argument for injury prevention. J Trauma. 2003;54(1):66–70; discussion 70–71.
5. Sampalis JS, Denis R, Lavoie A, et al. Trauma care regionalization: A process-outcome evaluation. J Trauma. 1999;46(4):565–579; discussion 579–581.
6. Shults RA, Elder RW, Nichols JL, et al.; Task Force on Community Preventive Services. Effectiveness of multicomponent programs with community mobilization for reducing alcohol-impaired driving. Am J Prev Med.2009;37(4):360–371.
7. Spaite DW, Tse DJ, Valenzuela TD, et al. The impact of injury severity and prehospital procedures on scene time in victims of major trauma. Ann Emerg Med. 1991;20(12):1299–1305.
8. Surgeons AC. Verified Trauma Centers.http://www.facs.org. 2013.
9. McConnell KJ, Johnson LA, Arab N, et al. The on-call crisis: A statewide assessment of the costs of providing on-call specialist coverage. Ann Emerg Med. 2007; 49(6):727–733, e1–e18.
10. Green SM. Is there evidence to support the need for routine surgeon presence on trauma patient arrival? Ann Emerg Med. 2006;47(5):405–411.
11. Grossman MD, The role of emergency medicine physicians in trauma care in North America: Evolution of a specialty. Scand J Trauma Resusc Emerg Med. 2009;17:37.
12. Moore EE. Role of the acute care surgeon in the emergency department management of trauma. Ann Emerg Med. 2006;47(5):413–414.
13. Mayglothling J, Duane TM, Gibbs M, et al.; Eastern Association for the Surgery of Trauma. Emergency tracheal intubation immediately following traumatic injury: An Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 suppl 4):S333–S340.
14. Wampler D, Schwartz D, Shumaker J, et al. Paramedics successfully perform humeral EZ-IO intraosseous access in adult out-of-hospital cardiac arrest patients. Am J Emerg Med. 2012;30(7):1095–1099.
15. Hallet J, Lauzier F, Mailloux O, et al. The use of higher platelet: RBC transfusion ratio in the acute phase of trauma resuscitation: A systematic review. Crit Care Med. 2013;41(12):2800–2811.
16. Holcomb JB, Zarzabal LA, Michalek JE, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011; 71(2 suppl 3):S318–S328.
17. Kutcher ME., Kornblith LZ, Narayan R, et al. A paradigm shift in trauma resuscitation: Evaluation of evolving massive transfusion practices. JAMA Surg. 2013; 148(9):834–840.
18. Bickell WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–1109.
19. Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: Impact on in-hospital mortality. J Trauma. 2002;52(6):1141–1146.
20. Morrison CA, Carrick MM, Norman MA, et al. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: Preliminary results of a randomized controlled trial. J Trauma. 2011;70(3):652–663.
21. Shlamovitz GZ, Mower WR, Bergman J, et al. Poor test characteristics for the digital rectal examination in trauma patients. Ann Emerg Med. 2007;50(1):25–33, 33 e1.
22. Chu UB, Clevenger FW, Imami ER, et al. The impact of selective laboratory evaluation on utilization of laboratory resources and patient care in a level-I trauma center. Am J Surg. 1996;172(5):558–562; discussion 562–563.
23. Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17(1):11–17.
24. Barleben A, Jafari F, Rose J Jr, et al. Implementation of a cost-saving algorithm for pelvic radiographs in blunt trauma patients. J Trauma. 2011;71(3):582–584.
25. Kendall JL., Kestler AM, Whitaker KT, et al. Blunt abdominal trauma patients are at very low risk for intra-abdominal injury after emergency department observation. West J Emerg Med. 2011;12(4):496–504.
26. Rhodes CM, Smith HL, Sidwell RA. Utility and relevance of diagnostic peritoneal lavage in trauma education. J Surg Educ. 2011;68(4):313–317.
27. Quinn AC, Sinert R. What is the utility of the Focused Assessment with Sonography in Trauma (FAST) exam in penetrating torso trauma? Injury. 2011;42(5):482–487.
28. Holmes JF, Offerman SR, Chang CH, et al. Performance of helical computed tomography without oral contrast for the detection of gastrointestinal injuries. Ann Emerg Med. 2004;43(1):120–128.
29. Petrosoniak A, Engels PT, Hamilton P, et al. Detection of significant bowel and mesenteric injuries in blunt abdominal trauma with 64-slice computed tomography. J Trauma Acute Care Surg.2013;74(4):1081–1086.
30. Livingston DH, Lavery RF, Passannante MR, et al. Admission or observation is not necessary after a negative abdominal computed tomographic scan in patients with suspected blunt abdominal trauma: Results of a prospective, multi-institutional trial. J Trauma. 1998;44(2):273–280; discussion 280–282.
31. Brenner DJ. Slowing the increase in the population dose resulting from CT scans. Radiat Res. 2010;174(6):809–815.
32. Brenner DJ, Hall EJ. Cancer risks from CT scans: Now we have data, what next? Radiology. 2012;265(2):330–331.
33. Richards PJ, Summerfield R, George J, et al. Major trauma & cervical clearance radiation doses & cancer induction. Injury. 2008;39(3):347–356.
34. Cullinane DC, Schiller HJ, Zielinski MD, et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture–update and systematic review. J Trauma.2011;71(6):1850–1868.
35. Stassen NA, Bhullar I, Cheng JD, et al. Selective nonoperative management of blunt splenic injury: An Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 suppl 4): S294–S300.
36. Haukoos J, Trauma HD, Research, in Trauma: A comprehensive emergency medicine approach. In: Legome E. Shockley L, eds. Cambridge University Press; 2011:New York, NY: 677–687.
37. Simmons E, Hedges JR, Irwin L, et al. Paramedic injury severity perception can aid trauma triage. Ann Emerg Med. 1995;26(4):461–468.