Bernadine R. Donahue and Jay S. Cooper
We cannot deal with AIDS by making moral judgments or refusing to face unpleasant facts, and still less by stigmatizing those who are infected.
—Kofi Annan, former U.N. secretary general
![]() HUMAN IMMUNODEFICIENCY VIRUS
HUMAN IMMUNODEFICIENCY VIRUS
One of the greatest public health challenges of the latter half of the 20th century has been emergence of human immunodeficiency virus (HIV). Although the biology of the virus has been elucidated, diagnostic tests have been created, and effective drugs and care systems have been established, HIV remains an epidemic in the 21st century, particularly in the developing world. HIV infection, immunosuppression, and enhanced tumor growth characterize the acquired immunodeficiency syndrome (AIDS).
Nearly 30 million people have died of AIDS in the past 30 years,1 and despite gains that have been made, AIDS remains a health catastrophe in those parts of the world with little or no access to life-sustaining drugs. In 2009, the most recent year for which worldwide statistics are available, over 33 million people were living with AIDS (including 2.5 million children under the age of 15 years), 2.6 million people became infected, and 1.8 million people died of AIDS. Nearly 70% of these events occurred in Sub-Saharan Africa.1 Although the toll of HIV infection in North America can be viewed as relatively small in comparison to other parts of the world, the statistics remain concerning. In 2009, 1.5 million persons in North America were living with HIV and there were 25,000 deaths attributable to AIDS.1 Many HIV-infected persons in the United States do not receive optimal care: for some, the monetary cost of highly active antiretroviral therapy (HAART) is prohibitive, while for others, the toxicity associated with HAART is unbearable. Despite funded programs aimed at reducing the incidence of AIDS, 70,000 persons became infected with HIV in North America during 2009.1 Thus, even in such resource-rich areas as North America, AIDS is likely to remain a serious problem in the foreseeable future.
Human Immunodeficiency Virus and Malignancy
AIDS-associated malignancies are a well-recognized, not-infrequent, and potentially lethal consequence of the disease. Early in the epidemic, three types of malignancies showed a sufficiently increased incidence that they qualified as AIDS-defining conditions when they occurred in conjunction with HIV infection: Kaposi’s sarcoma (KS), non-Hodgkin lymphoma (NHL), and carcinoma of the cervix. In 1981, the appearance of KS in young homosexual men heralded the association of tumors and HIV infection.2 Intermediate- or high-grade B-cell lymphomas in HIV-infected individuals were classified as AIDS-defining events in 1985.3 Cervical carcinoma was recognized in 1993 as an AIDS-defining illness for HIV-infected women.4 Data reported from the AIDS Cancer Match Registry Study (including more than 300,000 adult persons with HIV/AIDS) demonstrated the expected increased relative risks of developing the three AIDS-defining tumors,5 however, in addition, this study as well as others suggested an increase in some other tumors, such as Hodgkin lymphoma, anal carcinomas, skin cancers, and prostate cancer, as well as unusual pediatric age malignancies in HIV-infected children.6,7,8,9–10
By 1993, the Centers for Disease Control (CDC) dropped the requirement of an AIDS-defining malignancy or other overt illnesses for a diagnosis of AIDS4; however, the burden of cancer in the HIV-infected population remains a substantial and potentially growing problem. The raw numbers from the HIV/AIDS Cancer Match Study and the CDC reflect the overall decrease in AIDS-defining neoplasms over the past decades: from 1991–1995 to 2001–2005, the estimated number of AIDS-defining cancers decreased from 34,587 to 10,325, whereas non-AIDS-defining cancers increased from 3,193 to 10,059,11 and this finding is corroborated by multiple other datasets.8,12 It is likely that this trend will continue as the HIV-infected population ages,13–14,15–17,18,19 and given that the AIDS population in the United States expanded fourfold from 1991 to 2005 primarily because of an increase in the number of people aged 40 years or older,11 cancer in the setting of HIV infection will continue to require our attention.
This chapter subsequently is organized by histologic type of HIV-associated malignancy. Epidemiology, patterns of disease, pathology, diagnostic evaluation, treatment, and prognosis are included. The use of radiation as it relates specifically to each malignancy in the setting of HIV is discussed. For additional details regarding the delivery of radiation, the chapters in this text addressing each specific neoplasm should be consulted.
It is of historical interest to note that in June 2001, the International Atomic Energy Agency issued a monograph, “The Role of Radiotherapy in the Management of Cancer Patients Infected by Human Immunodeficiency Virus (HIV),” which, given the paucity of data and the immense scope of the problem, attempted to provide some guidance for the treatment of HIV-associated malignancies.20 At the time, AIDS was an almost uniformly fatal disease, and it was against this background that the authors stated that for HIV-associated malignancies:
[T]he usual oncological rules of practice do not apply: cure at any cost is not a sensible option. Very often the best decision is simply to treat with the simplest, most effective, palliative regimen available. Any decision to treat radically has to be tempered by the realisation that the patient’s life span will be limited, regardless of the success or failure of the treatment for the malignant disease. Nowhere in oncology is an individualised approach to decision-making more important than in HIV oncology… for a patient with asymptomatic malignancy and HIV infection, active observation is a perfectly reasonable policy.20
Fortunately, a decade later, the outlook for HIV-infected persons is much improved, and the era of therapeutic nihilism has past. There is now solid rationale for including patients with HIV and cancer in clinical trials. The Cancer Therapy Evaluation Program has advised researchers that individuals known to be HIV positive should not be arbitrarily excluded from participation in clinical cancer treatment trials, and the National Cancer Institute has advocated that persons with HIV and cancer should only be excluded from cancer trials if there is a scientific reason for doing so.21 In general, for persons with HIV, the procedure is to attempt to utilize “standard” stage-directed treatments for each malignancy discussed. However, the authors do so with the major caveat that the “standard” treatment may need to be modified based on the immunologic status, the viral load, coexisting opportunistic infections, and comorbidities in any given individual.
Treatment-related toxicity will continue to be a concern as HIV infection yields to better therapies and the incidence of unrelated tumors increases. Laboratory data suggest that certain protease inhibitors may increase radiosensitivity; however, this has not been borne out in the clinic. See et al.22 compared the toxicity rates of radiation therapy in patients who were taking or not taking a second-generation protease inhibitor and observed no difference. Baeyens et al.23 demonstrated heightened sensitivity of HIV-infected T lymphocytes to damage from radiation, but the in vivo data are not uniformly supportive. Kaminuma et al.24 concluded that radiotherapy is safe, but HIV infection was associated with earlier onset (i.e., with lower dose) and more severe acute skin and mucosal toxicity. Mallik et al.,25 in a review of the literature, concluded that local control and disease-specific survival are not affected by HIV infection if the CD4 count exceeds 200 cells per cubic millimeter. This appears to be true even for tumors that are treated with relatively aggressive combinations of chemotherapy and radiation, such as carcinomas of the anal canal or of the head and neck region.26,27 Thus, in general, it appears that curative radiation therapy (with or without chemotherapy as the extent of disease dictates) provides disease control without imparting a degree of toxicity substantially beyond that traditionally seen in HIV-uninfected individuals. Nevertheless, the myelosuppressive nature of some of the agents incorporated into HAART regimens and the neurotoxicity of others raise concern when radiation therapy is being delivered to large volumes of bone marrow or over the central nervous system (CNS).28 If we are to continue to improve the outcome of HIV-infected persons with malignancies, close monitoring for toxicity, attention to immunologic parameters, and a strong emphasis on supportive care are essential when treating such patients with standard treatments.
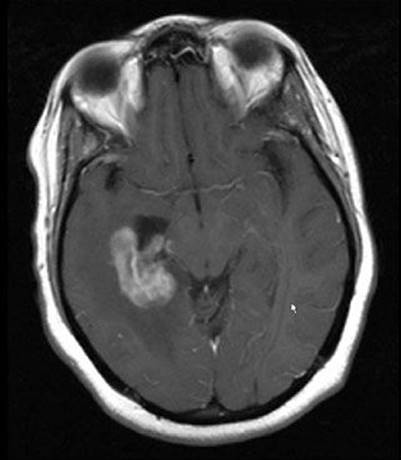
FIGURE 34.1. Example of periventricular location of human immunodeficiency virus–associated primary central nervous system lymphoma.

![]() LYMPHOMA
LYMPHOMA
The incidence of lymphoma in association with HIV infection was reported as approximately 60 to 100 times greater than expected in the general population during the early years of the epidemic.29,30 Although primary central nervous system lymphoma (PCNSL) was one of the initial CDC-approved criteria for a diagnosis of AIDS,31 the inclusion of systemic high-grade B-cell NHL did not occur until 1985.3 Its incidence was shown to correlate with the duration of immunosuppression, CD4 count 1 year prior to the diagnosis of NHL, and B-cell stimulation.32 Epstein-Barr virus (EBV) was implicated in its etiology by the finding of anti-EBV immunoglobulins and circulating EBV-infected B cells in the setting of HIV.33 Where available, HAART appears to have decreased the incidence of both PCNSL and NHL, most likely secondary to an overall decrease in the proportion of patients with low CD4 counts.12
Primary Central Nervous System Lymphoma
Epidemiology and Risk Factors
At its height, the incidence of NHL originating in the brain without evidence of systemic involvement in persons with AIDS was 3,600-fold higher than in the general population.34 HAART has decreased the high risk of developing this disorder in persons who are infected with HIV.35
Patterns of Disease
In most patients who have HIV-associated PCNSL, the diagnosis is suggested by the onset of headaches or a change in mental status.36,37 Unfortunately, PCNSL can be clinically and radiographically indistinguishable from other pathologic processes in HIV-infected patients. Neurocognitive dysfunction in HIV-infected patients is associated with a long differential diagnosis, including PCNSL, toxoplasmosis, herpetic infections, cryptococcus, progressive multifocal leukoencephalopathy, neuroimmune reconstitution inflammatory syndrome (IRIS), and HIV-associated dementia, leukoencephalopathy, and demyelination. In general, the typical radiographic findings of PCNSL are that of multiple contrast-enhancing lesions, often, but not exclusively, in a periventricular location (Fig. 34.1).
Diagnostic Workup
Prior to HAART, it was common for an HIV-infected patient to present with a clinical and radiographic picture that could be consistent with either toxoplasmosis or PCNSL (Fig. 34.2). A negative toxoplasmosis titer did not eliminate the diagnosis of toxoplasmosis, and similarly a positive toxoplasmosis titer did not preclude the presence of PCNSL.38,39 Because there was a high incidence of toxoplasmosis in the HIV-infected population, the standard first-line treatment for an HIV-infected patient who developed a neurologic abnormality and had a radiographically visible brain lesion consistent with either toxoplasmosis or CNS lymphoma was the institution of antitoxoplasmosis antibiotics. During the first decade of the epidemic, it was common to administer empiric cranial radiation therapy in patients who did not manifest clinical or radiographic improvement by the second or third week of antitoxoplasmosis treatment. However, as our knowledge of the myriad HIV-related opportunistic infections expanded and the poor outcome with this empiric approach was recognized, the emphasis shifted to recommending biopsy for definitive diagnosis.40
Pathology and Prognostic Factors
The majority of PCNSL are B-cell large immunoblastic types, and EBV DNA is identifiable in nearly all cases.41 Polymerase chain reaction amplification of EBV DNA in the cerebrospinal fluid is usually positive in these patients and may become negative following treatment.42 In general, PCNSL is seen later in the course of HIV infection than is systemic NHL. Patients usually have CD4 counts <50 cells per cubic millimeter before PCNSL becomes evident.43 Although the overall median survival of these patients is poor, patients no older than 35 years who have Karnofsky performance scores of at least 80% tended to survive 5 to 6 months, rather than the 2 months typically observed in less favorable subgroups.44
General Management
It has been suggested that the institution of HAART may result in regression of existing PCNSL, but to date scant evidence exists to support this claim.45–47 Methotrexate-based chemotherapy with or without whole-brain radiation therapy (RT) has been shown to prolong the median survival in immunocompetent patients who have PCNSL,48 and when possible, this approach should be considered for patients with HIV. However, many HIV-infected patients who are sufficiently immunosuppressed to develop PCNSL may be unable to tolerate methotrexate. In the pre-HAART era, chemotherapy was evaluated for HIV-associated PCNSL, with median survival on the order of 2 months.49,50 More recently, in the HAART era, a subgroup of HIV-positive patients who can tolerate aggressive therapy consisting of either methotrexate with or without whole-brain radiotherapy, may achieve longer survivals, although their outcome remains worse than the median survival of 41 months reported in immunocompetent patients treated with methotrexate-based chemotherapy.48,51
Radiation
Cranial irradiation alone in the treatment of PCNSL was essentially palliative, produced short-lived clinical and radiographic evidence of tumor response, and resulted in mean overall survival of 2 months.36,44,49,52–54,55,56–57,58Recent intriguing data from Japan showed a survival rate of 64% at 3 years in a cohort of irradiated patients who were receiving HAART.59 Patients who received (or who were able to receive) doses 30 Gy or more fared better than those receiving lower doses. Unfortunately, one-third of persons who lived more than 12 months manifested leukoencephalopathy.
Although HAART appears to have decreased the incidence of HIV-associated PCNSL, it remains to be determined what the precise influence of aggressive antiretroviral treatment will be on the outcome of HIV-infected patients with PCNSL. However, it appears that survival is prolonged by HAART.60,61 There are anecdotal reports of regression of PCNSL with the institution of HAART and case reports of survival 2 years or more.45–47,62 Cohort data from Australia showed that in a subset analysis of 47 patients with biopsy-proven PCNSL, antiretroviral therapy with at least two agents along with RT was associated with better survival.63 In addition to immunomodulation by targeting HIV, attempts have been made to target EBV, which is nearly uniformly found in HIV PCNSL. Combinations of zidovudine, ganciclovir, and interleukin-2 were piloted by the AIDS Malignancy Consortium.64 The incidence of myelosuppression with this was high, and this remains an experimental approach.
FIGURE 34.2. Magnetic resonance image findings in a patient who presented with a change in mental status and was found to be human immunodeficiency virus positive. Differential diagnosis included primary central nervous system lymphoma; biopsy proved toxoplasmosis.
Systemic Non-Hodgkin Lymphoma
Epidemiology and Risk Factor
The incidence of NHL decreased with introduction of HAART, and this reflected the decreased number of HIV-infected persons with low CD4 counts.65 Although it appears that nonnucleoside transcriptase inhibitor–based HAART is as protective as protease inhibitor–based HAART and more protective than nucleoside analogs alone,66 cases of NHL among patients with multiclass antiretroviral resistance have been described as developing soon after newer-class antiretrovirals were initiated; this has raised the potential of IRIS-mediated NHL.67,68
Diagnosis
Rapidly developing adenopathy or constitutional B symptoms (fevers, unexplained weight loss, night sweats) are the most common presentations of HIV-associated systemic NHL. Nearly 75% of all patients will present with advanced-stage disease (stage III or IV), will manifest B symptomatology, and frequently have extranodal involvement.69 The most common histologic subtypes are high-grade B-cell lymphoma or Burkitt’s lymphoma; however, intermediate-grade (diffuse large cell type) lymphomas are not uncommon. As patients who have AIDS-NHL frequently have extranodal involvement, staging evaluation should include chest, abdomen, and pelvic computerized tomograms, bone marrow biopsy, and cerebrospinal fluid analysis.
Treatment
Treatment for AIDS-NHL lymphoma initially was based on high-dose chemotherapy regimens that proved to be toxic. Subsequently, therapy relied on attenuated doses of cytotoxic chemotherapy or standard-dose chemotherapy plus cytokine support; however, outcomes were still poor.70–74 Further study helped to define a regimen with high efficacy with acceptable toxicity using infusional cyclophosphamide, doxorubicin, and etoposide (CDE), and a multicenter trial employing this regimen showed that the median 1-year survival of 48% achieved with CDE was approximately twice as high as was achievable with previously standard regimens such as methotrexate with leucovorin rescue, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone.75 The advent of rituximab, an anti-CD20 antibody that has improved survival for patients with non-HIV-associated NHL, may improve the outcome in HIV-associated NHL. An AIDS Malignancies Consortium phase III trial that randomized patients to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) versus CHOP plus rituximab (R-CHOP) showed a higher complete response rate (57%) in the R-CHOP arm as compared with CHOP alone (47%); this, however, was not statistically significant and there was an increased risk of death from infection in the R-CHOP arm.76 A French trial also evaluated the safety and efficacy of R-CHOP for the treatment of AIDS-related NHL.77 This trial included 61 patients, two-thirds of whom achieved a complete response. The overall survival at 2 years was 75%, and although infections were seen, only one patient died from infection. The AIDS Malignancies Consortium (AMC034) trial randomized patients to EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) with concurrent rituximab versus EPOCH followed sequentially by rituximab.78,79 The patients who received concurrent rituximab achieved a complete response of 73%.
CNS prophylaxis with intrathecal chemotherapy has been controversial in the setting of high-grade NHL and HIV infection, but frequently was used, especially in patients with extranodal disease. However, it appears that patients who do not have EBV-infected tumors may not require such prophylaxis. The risk of CNS involvement was 10 times higher in patients who were EBV positive, as compared with those who were EBV negative.80 It may be that prophylaxis can be reserved for a selected subset of patients. The sustained-release formulation of intrathecal cytarabine may have an important role to play in both the prophylaxis and treatment of CNS meningeal involvement.81
A previously unrecognized form of lymphoma, called primary effusion or body cavity lymphoma, was identified in HIV-infected patients during the second decade of the epidemic.82 It appeared to be associated with human herpesvirus-8,83 and it accounted for approximately 4% of AIDS-related NHL.84 Typically, such patients present with an effusion in a body cavity, in the absence of widespread lymphadenopathy. The effusion contains numerous atypical lymphoid cells with a plasmacytoid appearance and an indeterminate (non-B or T cell) immunophenotype and clonal immunoglobulin heavy- and light-chain gene rearrangements. Multiple myeloma-1/interferon regulatory factor-4 protein expression can be used to differentiate primary effusion lymphoma from other lymphomas.85 Survival of patients who have primary effusion lymphoma remains very short, on the order of 2 to 5 months, even with aggressive therapy.
It is clear that with the availability of HAART, it became feasible to employ standard chemotherapy regimens, and a major question now is whether HAART should be administered in conjunction with, or after, chemotherapy. In general, it is recommended that zidovudine be avoided because of its myelosuppressive effects. The current thinking is that HIV-infected persons should be treated in the same aggressive fashion as noninfected persons, however, patients much be chosen carefully and monitored closely for toxicity.86,87
Radiation Therapy
The role of consolidative radiation therapy following systemic treatment in AIDS patients who have NHL has not been evaluated methodically. It had been suggested that radiation should be used as a consolidative boost in patients with bulky disease who have demonstrated slow or partial response to chemotherapy.88,89 More obviously, it also was indicated for palliation of bulky lesions and was used to provide palliative therapy for patients who develop lymphomatous meningitis. In lymphomatous meningitis, this approach was shown to result in a 60% to 70% clinical or cytological response, however, median time to progression was on the order of only 2 to 2.5 months.90,91
Results and Prognosis
Despite our best current therapies, patients who have AIDS-NHL generally have a poor overall survival. Early in the epidemic prognostic factors were identified that correlated with the length of survival.92–94 Patients who had bone marrow involvement, low Karnofsky performance status at diagnosis (<70%), low CD4 counts, or a prior diagnosis of AIDS had a median survival on the order of 4 months; those without these adverse features had a median survival of 11 months. Rossi et al.95 reported that the International Prognostic Index (IPI), a model designed to predict the outcome of NHL in general, is a reliable prognostic indicator of outcomes for patients who have AIDS-related NHL. Both the likelihood of complete response and median survival after treatment correlated appropriately with IPI score. Moreover, the IPI score correlated with the CD4 cell count, suggesting that the degree of immunodeficiency imparted by HIV infection influenced the aggressiveness of NHL. Ultimately, a prognostic model for systemic AIDS-related NHL treated in the era of highly active antiretroviral therapy was established based solely on the IPI and the CD4 count.96
NHL remains an important cause of morbidity and mortality in AIDS patients. Registry data from San Diego suggest an improvement in median survival for these patients from approximately 4 months to 9 months after the introduction of HAART,35 however, data from Kaiser Permanente in California demonstrate that HIV-infected patients with NHL in the HAART era continue to endure substantially higher mortality compared with HIV-uninfected patients with NHL, with 59% of HIV-infected patients dying within 2 years after NHL diagnosis as compared with 30% of HIV-uninfected patients.97 There is hope that newer targeted agents, such as rituximab, which has shown an improvement in survival for patients with non-HIV-associated NHL, will improve the outcome in HIV-associated NHL. It is of interest that in developing countries challenged by the lack of HAART, efficacious regimens employing dose-modified oral chemotherapy have been developed, which result in outcomes comparable to the pre-HAART experience in the United States.98
Hodgkin Lymphoma
The relative risk of developing Hodgkin lymphoma in the setting of HIV infection (HIV-HL) is increased as compared with the general population as demonstrated by a joint Danish and U.S study of 302,824 HIV-infected patients that showed a relative risk of 11.5.99 Although data from the Multicenter AIDS Cohort Study showed a stable incidence of this disease before and after the introduction of HAART,12 there is recent evidence that the incidence of this disease in the HIV-infected population increased after the introduction of HAART. Data from a large European cohort showed a rising standardized incidence ratio from 1983 through 2007, with multivariate analysis showing that HAART was associated with an increased risk of disease.18
The explanation for the rise in HIV-HL after combined antiretroviral therapy became widely available is unclear, however, one intriguing explanation involves the relationship between Reed-Sternberg cells and CD4 cells.100 The risk of Hodgkin lymphoma appears to peak when HAART reconstitutes the immune system and CD4 cells reach levels of 150 to 190 cells per cubic millimeter. It is postulated that Reed-Sternberg cells produce growth factors that increase the influx of CD4 cells, which in turn provide signals that cause the proliferation of Reed-Sternberg cells. If CD4 cells stimulate the growth of Reed-Sternberg cells, it would stand to reason that as HAART improves the CD4 cell count, more of these cells are available to stimulate growth of the cell associated with Hodgkin lymphoma.
Hodgkin lymphoma in HIV-infected individuals tends to be advanced, associated with B symptoms, and can present with unusual manifestations, such as presentation with a gastric or intracranial mass.101,102 In addition to the usual prognostic factors for Hodgkin lymphoma, low CD4 count and preexisting AIDS confer a worse prognosis.
In the past, only 50% of patients had a complete response following combination chemotherapy, and 2-year survival was on the order of 45%.103,104 How to integrate RT into the management of HIV-HL was also problematic. A report from M.D. Anderson Cancer Center suggested that radiation therapy was “appropriate” for approximately 50% of patients who had HIV-HL, usually in combination with chemotherapy; nevertheless, even in that series (with a median follow-up of 64 months), 5-year overall survival was only 54%.105
However, the outcome may be improving in the setting of HAART and combination chemotherapy.106 A phase II study in 59 patients with HIV-HL (52 of whom also received concurrent HAART) resulted in a complete response rate of 81% with the Stanford V regimen, although 3-year overall survival was only 51%.107 A more recent series from Germany reported a risk-adapted approach in 93 patients with HIV-HL.108 Patients with early-stage favorable Hodgkin lymphoma were treated with 2 cycles of adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) and 30 Gy involved field RT, those with unfavorable early-stage disease received 4 cycles of BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) and 30 Gy involved field, and those with advanced stage disease were treated with 6 to 8 cycles of BEACOPP. Importantly, BEACOPP was replaced with ABVD for patients with “far-advanced” HIV infection, and HAART was given concomitantly with chemotherapy. Early results are encouraging with a 1-year overall survival of 88%, although the outcome was worse for those patients with advanced stage disease. Additionally, of concern, even with careful attention to the risks of toxicity, four patients died of neutropenic sepsis.
In general, radiation therapy should be considered for HIV-HL for the same indications it is considered in non-HIV-associated Hodgkin lymphoma; however, there continues to be a paucity of data on its use and tolerance in this setting.
FIGURE 34.3. Purple, 1.5-cm nodular classic Kaposi’s sarcoma on the ankle of an elderly man. (From Krigel RL, Friedman-Kien AE. Kaposi’s sarcoma of AIDS: diagnosis and treatment. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. AIDS: etiology, diagnosis, treatment, and prevention, 2nd ed. Philadelphia: JB Lippincott, 1988, with permission.)

![]() KAPOSI’S SARCOMA
KAPOSI’S SARCOMA
Epidemiology and Risk Factors
At the start of the epidemic, AIDS often was identified by the diagnosis of KS, and KS in this setting became known as epidemic Kaposi’s sarcoma (EKS). People infected with HIV had at least a 20,000 times greater risk of developing KS than uninfected individuals.109 The discovery that HIV-infected gay or bisexual men were more likely than HIV-infected heterosexual men to develop KS was one of the first clues that KS or its etiologic agent might somehow be sexually transmitted.
With the introduction of progressively more effective antiretroviral therapies, the incidence of KS in the United States, as a component of AIDS, has diminished over time. A report from the International Collaboration on HIV and Cancer evaluating the cancer incidence from 23 prospective studies that included 47,936 HIV-seropositive individuals from North America, Europe, and Australia showed that the adjusted incidence rate for KS declined from 15.2 in the period of time from 1992 through 1996 to 4.9 between 1997 and 1999.110 A report from the Swiss HIV Cohort Study showed that the risk of developing KS declined by 66% during the 15 months after HAART was initiated (p = .001), as compared to the pre-HAART era.111
In December 1994, a herpes virus that appeared to be associated with the etiology of Kaposi’s sarcoma was identified.112 This was called human herpesvirus-8 (HHV-8) as well as KS-associated herpesvirus (KSHV). The virus was detected in both the epidemic (AIDS-related) and endemic (previously typical African) forms of KS, as well as classic (elderly men of Eastern European or Mediterranean ancestry) KS. In a 1996 report from the Multicenter AIDS Cohort Study, antibodies to HHV-8 were detected in 80% of HIV-infected men who subsequently went on to develop KS.113 This suggested that KS resulted from infection with HHV-8, rather than being a direct result of HIV itself, or to cytokines induced by the HIV virus; HIV produced the immunosuppression that facilitated HHV-8 expression as KS. HHV-8 has a large number of genes that can encode homologues of host genes, many of which are involved in angiogenesis and the cell cycle. At present, six major subtypes (called A, B, C, D, E, and F) of HHV-8 are recognized based on the specific amino acid sequences of the gene K1 (ORF-K1) of the virus and further divided into subgroups known as clades (e.g., A1, A2, A3). Additionally, increased levels of interleukin-6, which is thought to be an important growth factor in HHV-8 associated neoplasms, have been found in tissues affected by HHV-8.114
Patterns of Disease
KS is characterized by purplish lesions on the skin or mucosal surfaces. The lesions can be macular, plaque-like, or nodular, with or without associated lymphadenopathy or lymphedema (Figs. 34.3–34.5). At presentation, skin lesions can be either single or multiple and may cause pain, bleeding, or disfigurement. As involvement of lymph nodes and lymphatic spaces occurs, progressive edema can result. This is seen most commonly in lesions involving the lower extremity, the inguinal regions, the genitalia, and the face. Visceral KS typically involves the aerodigestive tracts. Oropharyngeal lesions can result in life-threatening airway obstruction. Pulmonary involvement can result in life-threatening respiratory failure.
Diagnostic Workup
In addition to inspection of all visible skin and mucosal surfaces, the likelihood of visceral KS is sufficiently high that endoscopic evaluation of the gastrointestinal (GI) tract is appropriate for any patient with GI symptoms. In any patient who develops KS as the first sign of AIDS, a more comprehensive workup for HIV should be undertaken: complete physical examination, blood count and chemistries including CD4 lymphocyte count and viral load, chest x-ray, tuberculin test, anergy screen, and screen for sexually transmitted diseases.
Pathology
It is generally agreed that KS is a neoplasm of mesenchymal origin, and the histologic diagnosis of KS requires the identification of both spindle cell and vascular elements within the lesion. The spindle-shaped cells look much like fibroblasts and are generally considered the neoplastic element. Overall, the appearance often is suggestive of slitlike embryonic vascular channels filled with red blood cells; however, red cells characteristically are also found mixed within the spindle cell framework of the tumor.
FIGURE 34.4. A: Epidemic Kaposi’s sarcoma of the foot before treatment. B: Same patient approximately 1.5 years after 30 Gy was delivered in 10 fractions over 2 weeks by 6-MeV electron beam therapy (with bolus).

FIGURE 34.5. A: Epidemic Kaposi’s sarcoma of the upper and lower lateral eyelids before treatment. B: Same patient approximately 1 month after 30 Gy was delivered in 10 fractions over 2 weeks by kilovoltage x-rays. An eye shield was used to protect the lens of the eye. Residual pigmentation is visible.

Treatment
Alternatives to or in Association with Radiation Therapy
EKS typically exhibits multifocal distribution at the time of presentation, and radiation therapy has played a smaller and smaller role in its management over time as better alternatives have been discovered. The role of HAART is now well established and appears to result in durable clinical response rates of over 60% of patients.115 In one prospective cohort study of good risk (confined to skin or nodes or minimal oral disease) KS, treatment with HAART alone allowed 74% of patients to survive systemic-treatment free for 5 years.116 It is important to recognize, however, that a small subset of patients may experience a worsening of symptoms when HAART is instituted. Bower et al.117 have reported that after commencing HAART, 6.6% of patients with HIV-associated KS developed progressive immune reconstitution inflammatory syndrome KS (i.e., a worsening in their clinical status), despite control of virologic and immunologic parameters, felt to be secondary to an immune response against a preexisting pathogen.117
Systemic chemotherapy has been used for patients with advanced disease. A concern had been that cytotoxic chemotherapy potentially could further compromise the immune system and accelerate the effects of HIV infection. Also, the standard doses of chemotherapy used for solid tumors often resulted in unacceptable morbidity for these patients.118,119 Consequently, treatment protocols were developed with low-dose chemotherapy to which epidemic KS was responsive.120 The regimen of ABV became the gold standard in the 1990s.
Liposomal daunorubicin and doxorubicin were approved subsequently by the U.S. Food and Drug Administration (FDA) for the treatment of EKS. Randomized studies comparing these drugs with the standard ABV showed at least comparable activity with a more favorable toxicity profile,121,122 and the liposomal drugs are generally now used as first-line therapy. Response rates of 25% with a median duration of 4 months can be expected.121,122
Paclitaxel is also approved for treatment. Toxicity is mild except for myelosuppression, which can be dealt with by growth factors when necessary.123,124 Gill et al.123 reported a 60% response rate (nearly all partial responses) with a 10-month median duration of response in one phase II trial. Although there are other active drugs available, such as VP-16, paclitaxel has become a common second- or third-line drug following the liposomal agents, due to its activity and acceptable toxicity.
Without HAART, patients often require ongoing therapy to control symptomatic KS. However, when HAART is available and decreases HIV RNA to undetectable levels, chronic chemotherapy frequently can be discontinued.125
Radiation Therapy
Radiation therapy can also be useful for palliation of pain, bleeding, or edema. Typically, small fields that include only the distressing lesion and a small margin are treated, either with superficial quality x-rays or low-energy electrons (and bolus). One of the more commonly used dose-fractionation schemes is 30 Gy in 10 fractions delivered over 2 weeks, which has resulted in substantial benefit without substantial toxicity for the typical patient.126 More than 90% of lesions respond to therapy and approximately 70% respond completely.127 Stelzer and Griffin’s128 prospective randomized trial demonstrated a correlation between dose and response within the tested range of 8 Gy in 1 fraction to 40 Gy in 20 fractions over 4 weeks. Greater dose was associated with a higher response rate, a lower incidence of residual pigmentation, and a longer duration of tumor control. However, these data should not be interpreted as a blanket recommendation for higher dose; rather, this suggests that doses should be tailored to individual patients’ needs. For patients who have far-advanced AIDS (where a briefer duration of palliation will suffice and the appearance of the lesion is not as important), a dose of 8 Gy in 1 fraction often is preferable.129 In contrast, relatively healthy patients, who are treated to improve their cosmetic appearance, should receive relatively higher doses. Even then, it is prudent to remember that some residual purple pigmentation remains in approximately 55% of treated patients.128
Gratifying short-term palliation of painful swollen extremities secondary to far advanced KS can be achieved by covering the limb in bolus (or placing it in a water bath) and delivering a single fraction of 8 Gy by parallel opposed photon portals.
Despite the relative sensitivity of KS to radiation, toxicity of treatment needs to be considered in every situation. Treatment of symptomatic oropharyngeal lesions with radiation has been problematic because of the high degree of radiation-induced mucositis that these patients develop even following low-dose radiation therapy.130 Palliation of symptomatic visceral or mucosal disease should nearly always be attempted first with chemotherapy and HAART. However, there are rare instances in which patients’ lesions have failed to respond to systemic chemotherapy or in which patients are unable to tolerate systemic chemotherapy. These patients may benefit from attenuated doses of local radiation to palliate bleeding or obstructive lesions. Piedbois et al.131 have reported 88% objective response and “good palliation of symptoms” following 10 to 20 Gy (2.5 Gy per fraction, 4 fractions per week) for delicate anatomic sites, such as the penis, the palms, oral mucosa, or the conjunctivae. In one report of 25 patients who had pulmonary lesions treated with radiation therapy (10.5–15 Gy at 1.5 Gy per fraction), subjective improvement was observed in nearly 90% of patients, although only one-third of patients survived for 3 months.132
![]() CERVICAL CANCER
CERVICAL CANCER
Epidemiology
Cervical carcinoma in the presence of HIV infection was accepted by the CDC as an AIDS-defining illness in 1993.4 The association between HIV and cervical cancer is complex and, although it has been clearly demonstrated that there is an increased incidence of preinvasive lesions in HIV-infected women, it is not clear that there is a substantially higher incidence of invasive disease.133 Some authors argued that since the introduction of HAART, there was little evidence to support the continued status of cervical cancer as an AIDS-defining illness, because cervical cancer did not appear to have a strong relationship to immune function as determined by CD4 count or responsiveness to HAART.134 However, recent reports from Africa, where the AIDS epidemic continues to rage, suggest a doubling of the risk of cervical cancer in women with AIDS as compared to African women without AIDS.135
Not surprisingly, there appears to be an association between human papilloma virus (HPV) infection and risk for cervical epithelial abnormalities in HIV-infected women.136,137–139 Data suggest that the introduction of HAART reduces the burden of HPV infection and intraepithelial neoplasia, which may in turn explain why rates of cervical cancer do not appear to have increased during the HAART era.140,141 A report on a prospective cohort of 286 HIV infected women who initiated HAART and were assessed semiannually for HPV infection and intraepithelial lesions showed that HAART initiation among adherent women was associated with a significant reduction in prevalence and incident detection of oncogenic HPV infection as well as a decreased prevalence and more rapid clearance of oncogenic HPV-positive intraepithelial lesions.142
Diagnosis
Routine gynecologic evaluation, including Papanicolaou (PAP) smears (and colposcopy when warranted), is the most successful means of detecting the dysplastic and in situ lesions (cervical intraepithelial neoplasia [CIN]) that give rise to invasive lesions. Unfortunately, HIV-infected women often presented with advanced-stage disease, and, thus, in addition to vaginal bleeding and dyspareunia, the disease frequently manifested with abdominal or back pain, weight loss, or palpable cervical adenopathy. During the early years of the AIDS epidemic, up to 50% of HIV-infected women were diagnosed as having stage III or IV disease when they first sought care, as compared to approximately 20% of noninfected women.143,144
Treatment
Treatment should be dictated by the extent of disease, taking into account the patient’s history of opportunistic infections and overall medical status. HIV-infected women treated with CIN should be approached in a fashion similar to non-HIV infected women, albeit with attention to the possible increased risk of complications.145 Patients who have early-stage, nonbulky disease are usually treated with a radical hysterectomy and pelvic lymph node dissection. Patients who have more advanced local-regional disease should be treated in the same fashion as their non-HIV-infected counterparts. Although previously this would have been radiation therapy alone, in one series of patients treated by radiation alone for advanced disease, 50% had no or minimal response to treatment, and at a median follow-up of 3 months all had progression of disease.146 In a series of 42 HIV-positive patients treated with definitive radiotherapy at Tata Memorial Hospital in India, only 22 patients completed the full course of RT; 50% achieved a complete response.147 It should be noted that when RT alone was used to treat cervical cancer, the rates of acute toxicity in HIV-infected women, particularly genitourinary, GI, and cutaneous toxicity, were observed to be higher than expected.148
The current standard of care for advanced cervical cancer is a combination of radiation therapy and cisplatin-based chemotherapy. At present there are insufficient data to demonstrate the routine feasibility or efficacy of this approach in the setting of AIDS; however, based on the tolerability of combined modality approaches in other AIDS-related malignancies and the evidence of improved outcome in advanced cervical cancer in the general population, it is believed that advanced cervical cancers in women who do not have a specific contraindication for chemotherapy should receive concurrent chemotherapy, external-beam radiotherapy, and an intracavitary boost. Patients who have hematogenously borne metastatic disease may benefit from palliative chemotherapy or radiation therapy in an effort to reduce symptoms such as pain and bleeding.
Results and Prognosis
Although there initially was concern over the ability to deliver standard treatment to HIV-infected patients with cervical carcinoma, available data suggest treatment can be delivered, albeit with a higher risk of complications and perhaps a worse outcome. In a study from the early years of the epidemic, 9 of 16 HIV-infected women who had cervical carcinoma died at a mean interval of only 9.2 months from diagnosis.146 Data on the outcomes with chemoradiation in the HAART era are lacking.
Prevention and early diagnosis of cervical abnormalities prior to the development of invasive cancer appear to be critical if the outcomes for patients are to improve. Prospective data from the Women’s Interagency HIV Study have confirmed that with close monitoring (i.e., PAP smears every 6 months) and appropriate intervention, the incidence of invasive cervical cancer among women with HIV is not higher than in HIV-negative women.149 The widespread use of the FDA-approved recombinant vaccine targeting HPV-16 and HPV-18 may further decrease the incidence of cervical cancer in the setting of HIV.
![]() ANAL CANCER
ANAL CANCER
Anal carcinomas, like cervical carcinomas, appear to be related to sexually transmitted HPV (primarily HPV-16), with anal intercourse being a risk factor.150 HIV-infected homosexual men have increased serum HPV DNA as compared with HIV-seronegative homosexual men.151 In one study, more than 60% of HIV-infected men with abnormal anogenital examinations were found to have squamous intraepithelial lesions on biopsy.152 How HAART has impacted the development of anal intraepithelial neoplasia and the subsequent development of invasive anal cancer is still being defined. Some authors reported that antiretroviral treatment did not protect against the development of premalignant or malignant lesions,153 while others reported that HAART decreased the prevalence of anal intraepithelial neoplasia in men with persistent HPV but did not affect the HPV.154 However, recent data from a large European cohort of people with HIV evaluating the incidence of non-AIDS defining cancers showed an elevated incidence of anal cancer during the pre-HAART era (1983–1995), the early HAART era (1996–2001), and the established HAART era (2002–2007) with similar standardized incidence ratios in all three periods.18 Thus, at present, it appears that HAART does not diminish the excess risk of anal cancer in the HIV-positive population.
Early-stage lesions may present as an incidental finding during excision of condylomata or hemorrhoids or may be identified when patients present with anal fissures. However, many patients with HIV anal carcinoma present with more advanced disease, and signs and symptoms may include perianal or rectal pain, tenesmus, bleeding, mucous drainage, or palpable groin nodes. Evaluation and workup should include digital rectal examination, inguinal node evaluation with biopsy or fine-needle aspiration of suspicious nodes, anoscopy, gynecologic examination in women, body computed tomography, and consideration of positron emission tomography scan.
Carcinoma in situ frequently can be approached with measures employed for the treatment of genital warts (i.e., topical podophyllin, topical 5-fluorouacil [5-FU], and laser therapy). In patients with low-grade, early-stage lesions (T1) without any evidence of nodal involvement, local excision with wide margins may be acceptable if the sphincter function is preserved. However, for the patients with HIV anal carcinoma who present with more advanced T stages or nodal involvement, if treatment is to be definitive, chemoradiation (with 5-FU/mitomycin-C and radiation therapy as the standard of care in immunocompetent persons) is the treatment of choice when possible. Unfortunately, the administration of mitomycin-C to patients with advanced HIV may be associated with the potential for severe myelosuppression and hemolytic uremic syndrome, thus limiting its use in some patients. In early series, there was evidence that patients with HIV infection required longer breaks from chemoradiation because of severe skin reactions and almost uniformly required chemotherapy dose reductions because of neutropenia.155,156–157These toxicities led to various attempts to render treatment more tolerable in the setting of HIV.
In the early years of the AIDS epidemic, HIV-infected patients who had anal carcinoma appeared to have a shorter survival and a higher incidence of local failure as compared with non-HIV infected persons.155,158 It appears that after the introduction of HAART, the outcome for persons with HIV-anal cancer improved.159 Recent series have shown that with modern management, including intensity-modulated radiation therapy and growth factor support, hematologic toxicity in HIV patient was similar to immunocompetent patients.160,161 Additionally, in selected patients, local control and survival rates may now approach those of the non-HIV population when 5-FU/mitomycin–based chemotherapy and full-dose RT is delivered.
Seo et al.162 showed that at a median follow-up of 3 years, 14 HIV-infected patients had no differences in overall survival (91%) as compared with immunocompetent patients; disease-specific survival and colostomy-free survival were also similar. There were no differences in acute and late toxicity profiles between the two groups. In a multicentric cohort study comparing 40 HIV-positive patients with 81 HIV-negative patients treated for anal cancer, the 5-year overall survival was 61% and 65%, respectively; however, the local control was only 38% in the HIV-positive patients as compared with 87% in the HIV-negative patients.163 In a series of 34 HIV-infected patients with anal carcinoma treated with various combinations of chemotherapy and irradiation, actuarial local control and overall survival at 3 years were 63% and 69%, respectively.26 A series reported by Fraunholz et al.160 from Germany described 21 HIV-positive patients who were receiving highly active antiretroviral therapy and who were treated with standard chemoradiation. Five patients required chemotherapy dose reduction and five patients required an interruption of RT. At a median follow-up of more than 4 years, the 5-year local control, cancer-specific, and overall survival rates were 59%, 75%, and 67%, respectively. There was one treatment-related death. The authors also noted that in the 3 to 7 weeks following completion of treatment, CD4 counts decreased, and one-third of patients experienced an increase in viral load. Thus, although standard chemoradiation should be considered in HIV-anal cancer, careful attention must be paid to immunologic parameters and risks of toxicity.
Newer strategies employing targeted agents, such as in an AIDS Malignancy Consortium phase II study employing cetuximab in addition to 5-FU, cisplatin, and radiation (1.8 Gy per fraction to a total of 45 Gy without a planned break), may improve the outcome of HIV-associated anal carcinoma.164 This trial has accrued 45 patients and results are pending. However, it may be that, given an etiology similar to cervical carcinoma, a key to improving outcome in anal cancer would be to employ similar strategies as are used for cervical cancer with prevention and early diagnosis of mucosal abnormalities.165 Rigorous surveillance for anal intraepithelial neoplasia with cytology and anoscopy in the population at risk should be considered,166 and there is hope that widespread vaccination for HPV would be a major public health advance against anal cancer.
![]() LUNG CANCER
LUNG CANCER
The higher incidence of smoking in the HIV population has been a confounding variable in studies reporting an increased incidence of lung cancer in persons with HIV infection (HIV-LC),167,168 however, after controlling for smoking, it appears that the rates of lung cancer are higher in HIV-infected than uninfected patients with large cohort studies and meta-analysis showing standardized incidence ratios of 2 to 4 for HIV-LC.15,169,170
The presentation of lung cancer in the HIV population is marked by young age at presentation (38–50 years) and advanced disease at diagnosis (75% of patients).171 The most common subtype of non–small cell lung cancer in this group has been reported to be adenocarcinoma.172
In the pre-HAART the median survival of HIV-LC was only 4 to 5 months.172,173–174 Case reports documented increased toxicity (particularly esophageal) in patients with HIV irradiated for lung cancer,175 however, larger series do not necessarily report increased toxicity,171 although doses of >60 Gy usually were not employed.
It is unclear as to whether combined antiretroviral therapy improves the outcome in these patients.176 The largest cohort study in the HAART era included 30 patients, 27 of whom presented with stage IIB to IV disease. Although patients diagnosed with very early-stage disease could undergo surgery, the median survival was only 5 months for those with advanced disease.177 How to incorporate antiretroviral drug regimens into lung cancer treatment regimens remains undefined. In general, an attempt should be made to deliver standard treatment based on the stage of the disease with the caveat that the treatment approach may need to be modified based on the patient’s immunological status, viral load, and coexisting opportunistic infections.
![]() HEAD AND NECK TUMORS
HEAD AND NECK TUMORS
Given the increased smoking rate and HPV infection rate in persons with HIV, it would not be surprising to see an increased incidence of both squamous cell and HPV-related head and neck tumors in HIV-infected persons. Persons with HIV-AIDS have a threefold higher prevalence of oral HPV infection than the non-HIV population, and thus we may anticipate an increased incidence of HPV-associated oropharyngeal cancers in this population.178 North American data provide some evidence that since the beginning of the HAART era, there has been an increased incidence of head and neck cancers in persons with HIV infection.16 A recent report of a large European cohort of HIV-infected persons documented a trend in increasing incidence from the pre-HAART era through the present, but this did not reach statistical incidence.18
A recent review of carcinomas arising in the head and neck region in HIV-positive patients indicates that patients with HIV-AIDS are at an increased risk of developing mucosal squamous cell carcinoma, nasopharyngeal carcinoma, lymphoepithelial carcinoma of the salivary gland, and Merkel cell carcinoma.179 This review also suggested that HIV-positive patients with these cancers present at a younger age, with more aggressive disease and worse prognosis compared to HIV-negative patients.
Case reports describing the treatment of head and neck malignancies in patients with HIV do not suggest the same increased mucosal sensitivity as is seen in patients with HIV-KS.180,181 Larger series appear to corroborate this. Sanfilippo et al.182 reported a series of 12 HIV-positive patients with squamous cell carcinoma of the head and neck who underwent irradiation. Median radiation dose was 66.4 Gy and median duration of treatment was 51 days. Nearly two-thirds of patients developed grade 3 toxicity, but only one patient developed grade 4 confluent moist desquamation. Similar results were reported by Klein et al.27 Twelve HIV-infected patients received a median dose of 68 Gy for squamous cell carcinomas of the head and neck; half also received chemotherapy. Local-regional control at 3 years was estimated to be 92% and survival was estimated at 78%. Nearly half the patients required treatment breaks of more than 10 days, however, no hospitalizations or treatment-related fatalities occurred. Toxicity appeared comparable to historical controls as did tumor control. However, it must be remembered that these patients were a relatively “healthy” group of HIV-infected persons, with all having Karnofsky performance status of 80 or higher and a median CD4 count of 460.
There is a paucity of data examining the tolerance and outcome in HIV-infected persons treated with combined chemoradiation; however, there is some hope that biologic agents such as cetuximab, a monoclonal antibody already approved for use in combination with radiation therapy for the treatment of locally or regionally advanced squamous cell carcinoma of the head and neck, may offer a viable option for HIV-infected patients with locally advanced disease.183
![]() LIVER CANCER
LIVER CANCER
Increased levels of hepatitis B and C coinfections in the HIV population may explain the higher standardized incidence ratios of hepatocellular carcinoma reported in both North American and European datasets,13,18 although the data are conflicting as to whether the incidence is higher for persons receiving combined antiretroviral therapy. The French have published on the feasibility of liver transplant for cirrhosis in persons with hepatitis and HIV,184 but data on the treatment of hepatocellular carcinoma in the setting of HIV are lacking. A recent report detailing serious adverse effects in an HIV-positive patient who was also coinfected with HBV and treated with sorafenib reminds us that we have much to learn about potential interactions between the newer targeted agents for this disease and HAART.185
![]() PROSTATE CANCER
PROSTATE CANCER
Recent data have shown an increase in the number of cases of prostate cancer in HIV-infected persons.11 From 1991–1995 to 2001–2005, estimated counts increased from 87 to 759 cancers, seemingly driven by growth and aging of the AIDS population. There are no data to support approaching prostate cancer any differently in an otherwise healthy HIV-infected man than in a non-HIV infected one.186–188 Pantanowitz et al.189 reported on 17 HIV-positive patients from multiple institutions treated for prostate cancer with various standard treatments, including surgery, hormonal therapy, and radiotherapy. No serious treatment-related side effects were reported. Ng et al.190 reported a series of 14 HIV-positive patients with prostate cancer treated with external beam radiation therapy with or without brachytherapy at St. Vincent’s Hospital in New York City. Following treatment, only one patient’s prostate-specific antigen (PSA) level remained above 1.1 ng/mL, the average CD4 count remained stable, and the viral load increased in only 2 of 14 patients. There were no unusual complications, and no infections related to treatment. Silberstein et al.191 reported on eight HIV-positive men who underwent robotic-assisted laparoscopic prostatectomy for treatment of prostate cancer. Preoperatively, all eight were on HAART and had undetectable viral loads. HIV-positive men required more perioperative transfusions and had a higher incidence of perioperative ileus as compared with HIV-negative men. The PSA level in all eight HIV-positive patients remained undetectable at the short median follow-up time of 2.6 months. Thus, although long-term treatment outcomes in HIV-positive patients remain uncertain, early results suggest acceptable response rates and toxicities in HIV-negative patients treated with standard treatments.
![]() PEDIATRIC MALIGNANCIES
PEDIATRIC MALIGNANCIES
A major triumph in the war against AIDS has been the virtual elimination of the vertical transmission of HIV in the developed world. However, in Africa, 1,000 babies still acquire the virus every day,192 an appalling figure given that it has been more than 15 years since the first study that demonstrated the efficacy of zidovudine in reducing mother-to-child transmission of the virus.193 Children who go on to develop AIDS appear to be at increased risk of developing tumors,6 particularly NHL (Burkitt’s lymphomas accounted for the most common histologic subtype), but also including leiomyosarcomas, primary CNS lymphomas, and KS.194–195,196 The Pediatric Oncology Group reported 28 NHL, 4 B-cell acute lymphoblastic leukemias, 1 Hodgkin lymphoma, 8 leiomyosarcomas, 1 hepatoblastoma, and 1 schwannoma in a cohort of HIV-infected children.197 Hopefully, the prevention of pediatric HIV ultimately will lead to a further decline in these cancers.
It appears that HAART has decreased the incidence of pediatric HIV malignancies.198,199 In a study of more than 5,000 children who were under 15 years of at the time they were diagnosed as having AIDS, cancer was 40 times more frequent in children with HIV than in the general population in the pre-HAART era, as compared with 17 times more frequent in the HAART era.200
![]() SUMMARY
SUMMARY
HIV infection fosters immunosuppression, and immunosuppression fosters the appearance of malignancies. Progress requires therapies that counteract the immunosuppression associated with HIV infection. HAART appears to have decreased the incidence of KS and PCNSL; however, there appears to be an increase in many non-AIDS-defining malignancies in HIV-infected persons in the era of HAART. Data regarding the influence of HAART on prognosis of malignancies remain difficult to interpret, with some studies claiming improved survival and others claim no influence. Furthermore, even in areas where HAART is widely available, individual patients may not access or comply with treatment, and, if general adherence to or effectiveness of therapy is poor, the overall immune status of the population will remain low, and, consequently, there will be no decline in the incidence of HIV-associated malignancies. Although HAART has dramatically changed the face of AIDS, major challenges, not the least of which is the emergence of viral resistance, unfortunately ensure that improvements in the treatment of AIDS-associated malignancies will continue to be needed. Ultimately, the key to eradicating AIDS-associated malignancies will lie in the prevention of HIV transmission.
![]() REFERENCES
REFERENCES
1. 2009 AIDS epidemic update. Available at: http://www.unaids.org/en/dataanalysis/epidemiology/2009aidsepidemicupdate/.
2. Centers for Disease Control. Kaposi’s sarcoma and pneumocystis pneumonia among homosexual men—New York City and California. Morb Mortal Wkly Rep 1981;30:305–308.
3. Centers for Disease Control. Revision of the case definition of acquired immunodeficiency syndrome for national reported: United States. Ann Intern Med 1985;103:402–403.
4. Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA 1993;269:729–730.
5. Frisch M, Biggar RJ, Engels EA, et al. For the AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. JAMA 2001;85:1736–1745.
6. Biggar RJ, Frisch M, Goedert JJ. For the AIDS-Cancer Match Registry Group. Risk of cancer in children with AIDS. JAMA 2000;284:205–209.
7. Burgi S, Brodine S, Wegner S, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer 2005;104:1505–1511.
8. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008;123:187–194.
9. Hessol NA, Katz MH, Liu JY, et al. Increased incidence of Hodgkin’s disease in homosexual men with HIV infection. Ann Intern Med 1992;117:309–311.
10. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans. Human immunodeficiency viruses and T-cell lymphotropic viruses, vol. 67. Lyon (France): IARC, 1996.
11. Shiels MS, Pfeiffer RM, MH Gail, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103:753–762.
12. Seaberg EC, Wiley D, Martínez-Maza O, et al. Cancer incidence in the multicenter AIDS cohort study before and during the HAART era: 1984 to 2007. Cancer 2010;116:5507–5516.
13. Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr2009;52:203–208.
14. Clifford GM, Polesol J, Rickenbach M, et al. Cancer in the Swiss HIV cohort study: association with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005;97:425–432.
15. Engels EA, Brock MV, Chen J, et al. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol 2006;24:1383–1388.
16. Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS 2006;20:1645–1654.
17. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008;148:728–736.
18. Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 2008;27:884–890.
19. Silverberg MJ, Abrams DI. Do antiretrovirals reduce the risk of non-AIDS-defining malignancies? Curr Opin HIV AIDS 2009;4:42–51.
20. The role of radiotherapy in the management of cancer patients infected by human immunodeficiency virus (2001). Available at: http://www-pub.iaea.org/MTCD/publications/PDF/te_1224_prn.pdf.
21. Persad GC, Little RF, Grady C. Including persons with HIV infection in cancer clinical trials. J Clin Oncol 2008;26:1027–1032.
22. See AP, Zeng J, Tran PT, et al. Acute toxicity of second generation HIV protease-inhibitors in combination with radiotherapy: a retrospective case series. Radiat Oncol 2011;6:25.
23. Baeyens A, Slabbert JP, Willem P, et al. Chromosomal radiosensitivity of HIV positive individuals. Int J Radiat Biol 2010;86:584–592.
24. Kaminuma T, Karasawa K, Hanyu N, et al. Acute adverse effects of radiation therapy on HIV-positive patients in Japan: study of 31 cases at Tokyo Metropolitan Komagome Hospital. J Radiat Res2010;51:749–753.
25. Mallik S, Talapatra K, Goswami J. AIDS: a radiation oncologist’s perspective. J Cancer Res Ther 2010;6:432–441.
26. Hauerstock D, Ennis RD, Grossbard M, et al. Efficacy and toxicity of chemoradiation in the treatment of HIV-associated anal cancer. Clin Colorectal Cancer 2010;9:238–242.
27. Klein EA, Guiou M, Farwell G, et al. Primary radiation therapy for head-and-neck cancer in the setting of human immunodeficiency virus. Int J Radiat Oncol Biol Phys 2011;79:60–64.
28. Housri N, Yarchoan R, Kaushal A. Radiotherapy for patients with the human immunodeficiency virus: are special precautions necessary? Cancer 2010;116:273–283.
29. Beral V, Peterman T, Berkleman R, et al. AIDS-associated non-Hodgkin’s lymphoma. Lancet 1991;337:805–809.
30. Biggar RJ, Rabkin CS. The epidemiology of acquired immunodeficiency syndrome-related lymphomas. Curr Opin Oncol 1992;4:883–893.
31. Centers for Disease Control. Update on acquired immune deficiency syndrome (AIDS)—United States. Morb Mortal Wkly Rep 1981;31:507–514.
32. Grulich AE, Wan X, Law MG, et al. B-cell stimulation and prolonged immune deficiency are risk factors for non-Hodgkin’s lymphoma in people with AIDS. AIDS 2000;14:133–140.
33. Lane HC, Fauci AS. Immunologic abnormalities in the acquired immunodeficiency syndrome. Ann Rev Immunol 1985;3:477–500.
34. Cote TR, Manns A, Hardy CR, et al. Epidemiology of brain lymphoma among people with or without acquired immunodeficiency syndrome. J Natl Cancer Inst 1996;88:675–679.
35. Diamond C, Taylor TH, Aboumrad T, et al. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy. Cancer 2006;106:128–135.
36. Donahue B, Cooper J, Rush S, et al. Results of empiric radiotherapy for HIV associated primary CNS lymphomas. Int J Rad Oncol Biol Phys 1989;17(Suppl abstr 1028):223.
37. Donahue BR, Sullivan JW, Cooper JS. Additional experience with empiric radiotherapy for HIV-associated primary CNS lymphoma. Cancer 1995;76:328–332.
38. Loureiro C, Gill PS, Meyer PR, et al. Autopsy findings in AIDS-related lymphoma. Cancer 1988;62:735–739.
39. Porter SB, Sarde MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 1992;327:1640–1643.
40. Corn BW, Trock BJ, Curran WJ. Management of primary central nervous system lymphoma for the patient with acquired immunodeficiency syndrome. Cancer 1995;76:163–166.
41. Herndier BG, Kaplan LD, McGrath MS. Pathogenesis of AIDS lymphomas. AIDS 1994;8:1025–1049.
42. Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:1–10.
43. Levine AM. AIDS-associated malignant lymphoma. Med Clin North Am 1992;76:253–268.
44. Corn B, Donahue B, Rosenstock J, et al. Performance status and age as independent predictors of survival among AIDS patients with primary CNS lymphoma: a multivariate analysis of a multi-institutional experience. Cancer J Sci Am 1997;3:52–56.
45. Boyle B, Merrick S, Jacobs J. Primary CNS lymphoma and HAART. Int Conf AIDS 1998; 12:854 (abstr 42404).
46. Corales R, Taege A, Rehm S, et al. Regression of AIDS-related CNS lymphoma with HAART. Program and abstracts of the XIII International AIDS Conference. Durban, South Africa, July 9–14, 2000; abstr MoPpB1086.
47. McGowan JP, Shah S. Long-term remission of AIDS-related primary central nervous system lymphoma associated with highly active antiretroviral therapy. AIDS 1998;12:952–954.
48. Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol 2000;18:3144–3150.
49. Ambinder RF, Lee S, Curran WJ, et al. Phase II intergroup trial of sequential chemotherapy and radiotherapy for AIDS-Related primary central nervous system lymphoma. Cancer Ther 2003;1:215–221.
50. Jacomet C, Girard PM, Lebrette MG, et al. Intravenous methotrexate for primary central nervous system non-Hodgkin’s lymphoma in AIDS. AIDS 1997;11:1725–1730.
51. Diamond C, Taylor TH, Im T, et al. Highly active antiretroviral therapy is associated with improved survival among patients with AIDS-related primary central nervous system non-Hodgkin’s lymphoma. Curr HIV Res2006;4:375–378.
52. Baumgartner JE, Rachlin JR, Beckstead JH, et al. Primary central nervous system lymphomas: natural history: response to radiation therapy in 55 patients with acquired immunodeficiency syndrome. J Neurosurg 1990;73:206–211.
53. Corn BW, Donahue BR, Rosenstock JG, et al. Palliation of AIDS-related primary lymphoma of the brain: observations from a multi-institutional database. Int J Rad Oncol Biol Phys 1997;38:601–605.
54. Formenti SC, Gill PS, Lean E, et al. Primary central nervous system lymphoma in AIDS—results of radiation therapy. Cancer 1989;63:1101–1107.
55. Goldstein JD, Dickson DW, Moser FG, et al. Primary central nervous system lymphoma in acquired immune deficiency syndrome—a clinical and pathologic study with results of treatment with radiation. Cancer 1991;67:2756–2765.
56. Kasamon YL, Ambinder RF. AIDS-related primary central nervous system lymphoma. Hematol Oncol Clin North Am 2005;19:665–687.
57. Ling SM, Roach M, Larson DA, et al. Radiotherapy of primary central nervous system lymphoma in patients with and without human immunodeficiency virus—ten years of treatment experience at the University of California San Francisco. Cancer 1994;73:2570–2582.
58. Remick SC, Diamond C, Migliozzi JA, et al. Primary central nervous system lymphoma in patients with and without the acquired immunodeficiency syndrome—a retrospective analysis and review of the literature. Medicine1990;69:345–360.
59. Nagai H, Odawara T, Ajisawa A, et al. Whole brain radiation alone produces favourable outcomes for AIDS-related primary central nervous system lymphoma in the HAART era. Eur J Haematol2010;84:499–505.
60. Kreisl TN, Panageas KS, Elkin EB, et al. Treatment patterns and prognosis in patients with human immunodeficiency virus and primary central system lymphoma. Leuk Lymphoma 2008;49:1710–1716.
61. Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS 2003;17:1787–1793.
62. Aboulafia DM, Puswella AL. Highly active antiretroviral therapy as the sole treatment for AIDS-related primary central nervous system lymphoma: a case report with implications for treatment. AIDS Patient Care STDS2007;21:900–907.
63. Newell E, Hoy JF, Cooper SG, et al. Human immunodeficiency virus-related primary central nervous system lymphoma: factors influencing survival in 111 patients. Cancer 2004;100:2627–2636.
64. Aboulafia DM, Ratner L, Miles SA, et al., and AIDS Associated Malignancies Clinical Trials Consortium. Antiviral and immunomodulatory treatment for AIDS-related primary central nervous system lymphoma: AIDS Malignancies Consortium pilot study 019. Clin Lymphoma Myeloma 2006;6:399–402.
65. Besson C, Goubar A, Gabarre J, et al. Changes in AIDS-related lymphomas since the era of highly active antiretroviral therapy. Blood 2001;98:2339–2344.
66. Stebbing J, Gazzard B, Mandalia S, et al. Antiretroviral treatment regimens and immune parameters in the prevention of systemic AIDS-related non-Hodgkin’s lymphoma. J Clin Oncol 2004;22:2177–2183.
67. Huhn GD, Badri S, Vibhakar S, et al. Early development of non-Hodgkin lymphoma following initiation of newer class antiretroviral therapy among HIV-infected patients—implications for immune reconstitution. AIDS Res Ther 2010;7:44.
68. Jaffe HW, De Stavola BL, Carpenter LM, et al. Immune reconstitution and risk of Kaposi sarcoma and non-Hodgkin lymphoma in HIV-infected adults. AIDS 2011;25:1395–1403.
69. Safai B, Diaz B, Schwartz J. Malignant neoplasms associated with human immunodeficiency virus infection. CA-A Cancer J Clin 1996;42:74–90.
70. Bermudez AM, Grant K, Rodvien R, et al. Non-Hodgkin’s lymphoma in a population with or at risk for acquired immunodeficiency syndrome: indications for intensive chemotherapy. Am J Med1989;86:71–76.
71. Kaplan LD, Straus DJ, Testa MA, et al. Low-dose compared with standard-dose m-BACOD chemotherapy for non-Hodgkin’s lymphoma associated with human immunodeficiency virus infection. N Engl J Med 1997;336:1641–1648.
72. Levine AM, Espina BE, Tulpule A, et al. Low dose mBACOD with concomitant dideoxycytidine (ddC): an effective regimen in AIDS-related lymphoma. Blood 1993;82(Suppl 1):A1531.
73. Levine AM, Sullivan-Halley J, Pike MC, et al. Human immunodeficiency virus-related lymphoma. Prognostic factors predictive of survival. Cancer 1991;68:2466–2472.
74. Levine AM, Wernz JC, Kaplan L, et al. Low dose chemotherapy with central nervous system prophylaxis and zidovudine maintenance in AIDS-related lymphoma. JAMA 1991;226:84–88.
75. Sparano JA, Lee S, Chen M, et al. Phase II Trial of infusional cyclophosphamide, doxorubicin and etoposide (CDE) in HIV-associated non-Hodgkin’s lymphoma: an Eastern Cooperative Oncology Group Trial (E1494). J Acquir Imune Defic Syndr 1999;21:A39 (abstr 120).
76. Kaplan LD, Lee JY, Ambinder RF, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood 2005;106:1538–1543.
77. Boue F, Gabarre J, Gisselbrecht C, et al. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin’s lymphoma. J Clin Oncol 2006;24:4123–4128.
78. Levine AM, Lee J, Kaplan L, et al. Efficacy and toxicity of concurrent rituximab plus infusional EPOCH in HIV-associated lymphoma: AIDS Malignancy Consortium Trial 034. J Clin Oncol 26(Suppl abstr 8527):460S.
79. Sparano JA, Lee JY, Kaplan LD, et al. Rituximab plus concurrent infusional EPOCH is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood 2010;115:3008–3016.
80. Cingolani A, Gastaldi R, Fassone L, et al. Epstein-Barr virus infection is predictive of CNS involvement in systemic AIDS-related non-Hodgkin’s lymphoma. J Clin Oncol 2000;18:3325–3330.
81. Mazhar D, Stebbing J, Bower M. Non-Hodgkin’s lymphoma and the CNS: prophylaxis and therapy in immunocompetent and HIV-positive individuals. Expert Rev Anticancer Ther 2006;6:335–341.
82. Ansari MQ, Dawson DB, Nadon R, et al. Primary body cavity-based AIDS-related lymphomas. Am J Clin Pathol 1996;105:221–229.
83. Jaffe ES. Primary body cavity-based AIDS-related lymphomas. Evolution of a new disease entity. Am J Clin Pathol 1996;105:141–143.
84. Simonelli C, Spina M, Cinella R, et al. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J Clin Oncol 2003;21:3948–3954.
85. Carbone A, Gloghini A, Cozzi MR, et al. Expression of MUM/IRF4 selectively clusters with primary effusion lymphoma among lymphomatous effusions: implications for disease histogenesis and pathogenesis. Br J Haemetol2000;111:247–257.
86. Spano JP, Costagliola D, Katlama C, et al. AIDS-related malignancies: state of the art and therapeutic challenges. J Clin Oncol 2008;26:4834–4842.
87. Sparano, JA. HIV-associated lymphoma: the evidence for treating aggressively but with caution. Curr Opin Oncol 2007;19:458–463.
88. Cooper JS, Donahue BR. The Swift article reviewed [editorial]. Oncology 1997;11:697–702.
89. Swift PS. Radiation therapy for malignancies in the setting of HIV disease. Oncology 1997;11:683–694.
90. Chamberlain MC, Dirr L. Involved-field radiotherapy and intra-ommaya methotrexate/cytarabine in patients with AIDS-related lymphomatous meningitis. J Clin Oncol 1993;11:1978–1984.
91. Donahue B, Steinfeld AD, Torrey MJ, et al. Lymphomatous meningitis in HIV-associated non-Hodgkin’s lymphoma: an unexpected pattern of relapse. In: Proceedings of the Ninth International Congress on Anti-Cancer Treatment. Paris: 1999.
92. Knowles DM, Chemulak GA, Subar M, et al. Lymphoid neoplasia associated with the acquired immunodeficiency syndrome (AIDS). Ann Int Med 1988;108:744–753.
93. Levine AM, Gill PS, Meyer PR, et al. Retrovirus and malignant lymphoma in homosexual men. JAMA 1985;254:1921–1925.
94. Lowenthal DA, Strauss DJ, Campbell SW, et al. AIDS-related lymphoid neoplasia: the Memorial Hospital experience. Cancer 1988;61:2325–2337.
95. Rossi G, Donisi A, Casari S, et al. The international prognostic index can be used as a guide to treatment decisions regarding patients with human immunodeficiency virus-related systemic non-Hodgkin lymphoma. Cancer1999;86:2391–2397.
96. Bower M, Gazzard B, Mandalia S, et al. A prognostic index for systemic AIDS-related non-Hodgkin lymphoma treated in the era of highly active antiretroviral therapy. Ann Intern Med 2005;143:265–273.
97. Chao C, Xu L, Abrams D, et al. Survival of non-Hodgkin lymphoma patients with and without HIV infection in the era of combined antiretroviral therapy. AIDS 2010;24:1765–1770.
98. Mwanda WO, Orem J, Fu P, et al. Dose-modified oral chemotherapy in the treatment of AIDS-related non-Hodgkin’s lymphoma in East Africa. J Clin Oncol 2009;27:3480–3488.
99. Watson J. Investigation reveals Hodgkin’s disease might be an AIDS-defining condition. Hem Onc Today 2001;2:6.
100. Levine AM. Hodgkin lymphoma: to the HAART of the matter. Blood 2006;108:3630.
101. Doweiko J, Dezube BJ, Pantanowitz L. Unusual sites of Hodgkin’s lymphoma: case 1. HIV-associated Hodgkin’s lymphoma of the stomach. J Clin Oncol 2004;22:4227–4228.
102. Figueroa BE, Brown JR, Nascimento A, et al. Unusual sites of Hodgkin’s lymphoma: case 2. Hodgkin’s lymphoma of the CNS masquerading as a meningioma. J Clin Oncol 2004;22:4228–4230.
103. Ames ED, Conjalka MS, Goldberg AF, et al. Hodgkin’s disease and AIDS: twenty-three new cases and a review of the literature. Hematol Oncol Clin North Am 1991;5:343–356.
104. Pelstring RJ, Zellmer RB, Sulak LE, et al. Hodgkin’s disease in association with human immunodeficiency virus infection: pathologic and immunologic features. Cancer 1991;67:1865–1873.
105. Tsimberidou AM, Sarris AH, Medeiros LJ, et al. Hodgkin’s disease in patients infected with human immunodeficiency virus: frequency, presentation and clinical outcome. Leuk Lymphoma 2001;41:535–544.
106. Biggar RJ, Jaffe ES, Goedart JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood 2006;108:3786–3791.
107. Spina M, Gabarre J, Rossi G, et al. Stanford V regimen and concomitant HAART in 59 patients with Hodgkin disease and HIV infection. Blood 2002;100:1984–1988.
108. Hentrich M, Berger M, Hoffman C, et al. HIV-associated Hodgkin’s lymphoma (HIV-HL): results of a prospective multicenter trial. J Clin Oncol 2010;28(15 Suppl):8035.
109. Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet 1990;335:123–128.
110. International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. JNCI 2000;92:1823–1830.
111. Ledergerber B, Egger M, Erard V, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss cohort study. JAMA 1999;282:2220–2226.
112. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994;266:1865–1869.
113. Gao SJ, Kingsley L, Hoover DR, et al. Seroconversion to antibodies against Kaposi’s sarcoma—associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med 1996;335:233–241.
114. Cannon M, Cesarman E. Kaposi’s sarcoma-associated herpes virus and acquired immunodeficiency-related malignancy. Semin Oncol 2000;27:409–419.
115. Aversa SM, Cattelan AM, Salvango L, et al. Treatments of AIDS-related Kaposi’s sarcoma. Crit Rev Oncol Hematol 2005;53:253–265.
116. Bower M, Weir J, Francis N, et al. The effect of HAART in 254 consecutive patients with AIDS-related Kaposi’s sarcoma. AIDS 2009;23:1701–1706.
117. Bower M, Nelson M, Young AM, et al. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J Clin Oncol 2005;23:5224–5228.
118. Gelmann E, Longo D, Lane H, et al. Combination chemotherapy of disseminated Kaposi’s sarcoma in patients with the acquired immune deficiency syndrome. Am J Med 1987;82:456–461.
119. Laubenstein LJ, Krigel RL, Odajnky CM, et al. Treatment of epidemic Kaposi’s sarcoma with etoposide or a combination of doxorubicin, bleomycin, and vincristine. J Clin Oncol 1984;2:1115–1120.
120. Gill PS, Rarick MU, McCutchan JA, et al. Systemic treatment of AIDS-related Kaposi’s sarcoma: results of a randomized trial. Am J Med 1991;90:427–433.
121. Gill PS, Wernz J, Scadden D, et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcoma. J Clin Oncol 1996;14:2353–2364.
122. Northfelt D, Stewart S. DOXIL (pegylated liposomal doxorubicin) as first-line therapy of AIDS-related Kaposi’s sarcoma (KS): integrated efficacy and safety results from two comparative trials. Abst 4th Conf Retro Opportun Infect 1997;736:200.
123. Gill PS, Tulpule A, Espina BM, et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcoma. J Clin Oncol 1999;17:1876–1883.
124. Gill PS, Tulpule A, Reynolds T, et al. Paclitaxel (Taxol) in the treatment of relapsed or refractory advanced AIDS-related Kaposi’s sarcoma. Proc ASCO 1996;15:A854.
125. Volm M, Wernz J. Patients with advanced AIDS-related Kaposi’s sarcoma (EKS) no longer require systemic chemotherapy after introduction of effective antiretroviral therapy. Proc ASCO 1997;16:A46.
126. Cooper JS, Steinfeld AS, Lerch IA. The prognostic significance of residual pigmentation following radiotherapy of epidemic Kaposi’s sarcoma. J Clin Oncol 1989;7:619–621.
127. Cooper J, Steinfeld A, Lerch, I. Intentions and outcomes in the radiotherapeutic management of epidemic Kaposi’s sarcoma. Int J Radiation Oncology Biol Phys 1991;20:419–422.
128. Stelzer KJ, Griffin TW. A randomized prospective trial of radiation therapy for AIDS-associated Kaposi’s sarcoma. Int J Radiat Oncol Biol Phys 1993;27:1057–1061.
129. Berson AM, Quivey JM, Harris JW, et al. Radiation therapy for AIDS-related Kaposi’s sarcoma. Int J Radiat Oncol Biol Phys 1990;19:569–575.
130. Cooper JS, Fried PR. Toxicity of oral radiotherapy in patients having AIDS. Arch Otolaryngol 1987;113:327–328.
131. Piedbois P, Frikha H, Martin L, et al. Radiotherapy in the management of epidemic Kaposi’s sarcoma. Int J Radiat Oncol Biol Phys 1994;30:1207–1211.
132. Meyer JL. Whole lung irradiation for Kaposi’s sarcoma. Ann J Clin Oncol 1993;16:372–376.
133. Chirenje ZM. HIV and cancer of the cervix. Best Pract Res Clin Obstet Gynaecol 2005;19:269–276.
134. Bower M, Mazhar D, Stebbing J. Should cervical cancer be an acquired immunodeficiency syndrome-defining cancer? J Clin Oncol 2006;24:2417–2419.
135. Brower V. AIDS-related cancers increase in Africa. J Natl Cancer Inst 2011;103:918–919.
136. Chaturvedi AK, Madeleine MM, Biggar RJ, et al. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009;101:1120–1130.
137. Sun XW, Kuhn L, Ellenbrock TV, et al. Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med 1997;337:1343–1349.
138. Varmuad SH, Kelley KF, Klein RS, et al. High risk of human papilloma virus infection and cervical squamous intraepithelial lesions among women with symptomatic human immunodeficiency virus infection. Am J Obstet Gynecol 1991;165:392–400.
139. Williams B, Darragh TM, Vranizar K, et al. Analysis of cervical human papilloma virus infection and risk of anal and cervical epithelial abnormalities in human immunodeficiency virus-infected women. Obstet Gynecol1994;83:205–211.
140. Heard I, Schmitz V, Costagliola D, et al. Early regression of cervical lesions in HIV-seropositive women receiving highly active antiretroviral therapy. AIDS 1998;12:1459–1464.
141. Minkoff H, Ahdieh L, Massa LS, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS 2001;15:2157–2164.
142. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis 2010;201:681–690.
143. Kahesa C, Mwaiselage J, Wabinga HR, et al. Association between invasive cancer of the cervix and HIV-1 infection in Tanzania: the need for dual screening. BMC Public Health 2008;8:262.
144. Maiman M, Fruchter RG, Guy L, et al. Human immunodeficiency virus infection and invasive cervical carcinoma. Cancer 1993;71:402–406.
145. Cuthill S, Maiman M, Fruchter RG. Complications after treatment of cervical intraepithelial neoplasia in women infected with the human immunodeficiency virus. J Reprod Med 1995;40:823–828.
146. Chadha M, Sood B, Stanson R. Patients with human immunodeficiency virus (HIV): infections and cervical neoplasia. Int J Rad Oncol Biol Phys 1984;30(S1):284.
147. Shrivastava SK, Engineer R, Rajadhyaksha S, et al. HIV infection and invasive cervical cancers, treatment with radiation therapy: toxicity and outcome. Radiother Oncol 2005;74:31–35.
148. Gichangi P, Bwayo J, Estimable B, et al. HIV impact on acute morbidity and pelvic tumor control following radiotherapy for cervical cancer. Gynecol Oncol 2006;100:405–411.
149. Massad LS, Seaberg EC, Watts DH, et al. Long-term incidence of cervical cancer in women with human immunodeficiency virus. Cancer 2009;115:524–530.
150. Frisch M, Glimelius B, Van Der Brule AJC, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med 1997;337:1350–1358.
151. Northfelt DW, Swift PS, Palefsky JM. Anal dysplasia: Pathogenesis, diagnosis and management. In: Krown SE, von Roenn JH, eds. Hematologic and oncologic aspects of HIV infection. Hematology Oncology Clinics of North America. Philadelphia: WB Saunders, 1996:117–118.
152. Muldrow ME, Orr LK, Douglas JM, et al. Intraepithelial neoplasia of the anogenital area in HIV infected homosexual men. J Acquir Immune Defic Syndr Hum Retrovirol 1997;14:A180 (abstr 10).
153. Berry JM, Palefsky JM, Welton ML. Anal cancer and its precursors in HIV-positive patients: perspectives and management. Surg Oncol Clin North Am 2004;13:355–373.
154. Wilkin TJ, Palmer S, Brudney KF, et al. Anal intraepithelial neoplasia in heterosexual and homosexual HIV-positive men with access to antiretroviral therapy. J Infect Dis 2004;190:1685–1691.
155. Cho DS, Shieh G, Kuber N, et al. Definitive radiotherapy in the combined modality treatment of HIV anal carcinoma. Proc ASCO 2001;20:150b (abstr 2350).
156. Holland JM, Swift PS. Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy. Radiology 1994;193:251–254.
157. Kim JH, Sarani B, Orkin BA, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum 2001;44:1496–1502.
158. Peddada AV, Smith PE, Rao AR. Chemotherapy and low-dose radiotherapy in the treatment of HIV-infected patients with carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 1997;37:1101–1105.
159. Barriger RB, Calley C, Cárdenes HR. Treatment of anal carcinoma in immune-compromised patients. Clin Transl Oncol 2009;11:609–614.
160. Fraunholz I, Weiss C, Eberlein K, et al. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin c for invasive anal carcinoma in human immunodeficiency virus-positive patients receiving highly active antiretroviral therapy. Int J Radiat Oncol Biol Phys 2010;76:1425–1432.
161. Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol 2007;25:4581–4586.
162. Seo Y, Kinsella MT, Reynolds HL, et al. Outcomes of chemoradiotherapy with 5-fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys 2009;75:143–149.
163. Oehler-Jänne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol 2008;26:2550–2557.
164. Cisplatin, fluorouracil, cetuximab, and radiation therapy in treating patients with HIV and stage I, stage II, or stage III anal cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT00324415?term=anal+cancer+and+HIV&rank=6.
165. Chiao EY, Giordano TP, Palefsky JM, et al. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis 2006;43:223–233.
166. Palefsky JM, Holly EA, Hogeboom CJ. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune Defic Syndr Hum Retrovirol 1997;14:415–422.
167. Cadranel J, Garfield D, Lavole A, et al. Lung cancer in HIV infected patients: facts, questions and challenges. Thorax 2006;61:1000–1008.
168. Levine AM, Seaberg EC, Hessol NA, et al. HIV as a risk factor for lung cancer in women: data from the Women’s Interagency HIV Study. J Clin Oncol 2010;28:1514–1519.
169. Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67.
170. Kirk GD, Merlo C, O’Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis 2007;45:103–110.
171. Spano JP, Massiani MA, Bentata M, et al. Lung cancer in patients with HIV Infection and review of the literature. Med Oncol 2004;21:109–115.
172. Tirelli U, Spina M, Sandri S, et al. Lung carcinoma in 36 patients with human immunodeficiency virus infection. The Italian Cooperative Group on AIDS and tumors. Cancer 2000;88:563–569.
173. Sridhar KS, Flores MR, Raub WA Jr, et al. Lung cancer in patients with human immunodeficiency virus infection compared with historic control subjects. Chest 1992;102:1704–1708.
174. Vyzula R, Remick SC. Lung cancer in patients with HIV-infection. Lung Cancer 1996;15:325–339.
175. Leigh BR, Lau DH. Severe esophageal toxicity after thoracic radiation therapy for lung cancer associated with the human immunodeficiency virus: a case report and review of the literature. Am J Clin Oncol 1998;21:479–481.
176. Bazoes A, Bower M, Powles T. Smoke and mirrors: HIV-related lung cancer. Curr Opin Oncol 2008;20:529–533.
177. Hakimian R, Fang H, Thomas L, et al. Lung cancer in HIV-infected patients in the era of highly active antiretroviral therapy. J Thorac Oncol 2007;2:268–272.
178. Gillison ML. Oropharyngeal cancer: a potential consequence of concomitant HPV and HIV infection. Curr Opin Oncol 2009;21:439–444.
179. Purgina B, Pantanowitz L, Seethala RR. A review of carcinomas arising in the head and neck region in HIV-positive patients. Patholog Res Int 2011;2011:469150.
180. Harris MA, Wise MS, Bonington A, et al. Case report: radical radiotherapy for early laryngeal cancer in a patient with human immunodeficiency virus: no evidence of increased toxicity. Br J Radiol2004;77:519–520.
181. Kao GD, Devine P, Mirza N. Oral cavity and oropharyngeal tumors in human immunodeficiency virus-positive patients: acute response to radiation therapy. Arch Otolaryngol Head Neck Surg1999;125:873–876.
182. Sanfilippo NJ, Mitchell J, Grew D, et al. Toxicity of head-and-neck radiation therapy in human immunodeficiency virus-positive patients. Int J Radiat Oncol Biol Phys 2010;77:1375–1379.
183. Rubinstein PG, Lindgren V, Setty S, et al. Durable complete remission induced by cetuximab monotherapy in a patient infected with HIV and diagnosed with recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2011;29:e222–225.
184. Tateo M, Roque-Afonso AM, Antonini TM, et al. Long-term follow-up of liver transplanted HIV/hepatitis B virus coinfected patients: perfect control of hepatitis B virus replication and absence of mitochondrial toxicity. AIDS2009;23:1069–1076.
185. Ozenne V, Gervais A, Peytavin G, et al. Suspected interaction between sorafenib and HAART in an HIV-1 infected patient: a case report. Hepatogastroenterology 2011;58:161–162.
186. Levinson A, Nagler EA, Lowe FC. Approach to management of clinically localized prostate cancer in patients with human immunodeficiency virus. Urology 2005;65:91–94.
187. O’Connor JK, Nedzi LA, Zakris EL. Prostate adenocarcinoma and human immunodeficiency virus: report of 3 cases and review of the literature. Clin Genitourin Cancer 2006;5:85–88.
188. Wosnitzer MS, Lowe FC. Management of prostate cancer in HIV-positive patients. Nat Rev Urol 2010;7:348–357.
189. Pantanowitz L, Bohac G, Cooley TP, et al. Human immunodeficiency virus-associated prostate cancer: clinicopathological findings and outcome in a multi-institutional study. BJU Int 2008;101:1519–1523.
190. Ng T, Stein NF, Kaminetsky J, et al. Preliminary results of radiation therapy for prostate cancer in human immunodeficiency virus-positive patients. Urology 2008;72:1135–1138.
191. Silberstein JL, Parsons JK, Palazzi-Churas K, et al. Robot-assisted laparoscopic radical prostatectomy in men with human immunodeficiency virus. Prostate Cancer Prostatic Dis 2010;13:328–332.
192. UNICEF and partners report progress on preventing mother-to-child HIV transmission (2010). Available at: http://www.unicef.org/aids/index_57031.html.
193. Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med 1994;331:1173–1180.
194. Chadwick EG, Connor EJ, Hanson IC, et al. Tumors of smooth-muscle origin in HIV-infected children. JAMA 1990;263:3182–3184.
195. Epstein LG, DiCarlo FJ, Joshi VV, et al. Primary lymphomas of the central nervous system in two children with acquired immunodeficiency syndrome. Ann J Clin Pathol 1990;94:722–728.
196. Granovsky MO, Mueller BU, Nicholson HS, et al. Cancer in human immunodeficiency virus infected children: a case series from the Children’s Cancer Group and the National Cancer Institute. J Clin Oncol 1988;16:1729–1735.
197. Pollock BH, Jenson HB, Leach CT, et al. Risk factors for pediatric human immunodeficiency virus-related malignancy. JAMA 2003;289:2393–2399.
198. Kest H, Brogly S, McSherry G, et al. Malignancy in perinatally human immunodeficiency virus-infected children in the United States. Pediatr Infect Dis J 2005;24:237–242.
199. Nachman SA, Chernoff M, Gona P, et al. Incidence of noninfectious conditions in perinatally HIV-infected children and adolescents in the HAART era. Arch Pediatr Adolesc Med 2009;163:164–171.
200. Simard E, Shiels M, Bhatia K, et al. Long-term cancer risk among people diagnosed with AIDS during childhood. 18th Conference on Retroviruses and Opportunistic Infections. February 27–March 2, 2011. Boston. Abstract 82LB as reviewed by Mark Mascolini. Available at: http://www.natap.org/2011/CROI/croi_14.htm.