J.V. Anandan
LEARNING OBJECTIVES
Upon completion of the chapter, the reader will be able to:
1. Identify the primary reasons why some parasitic diseases may be more prevalent in the U.S. population.
2. Describe the treatment algorithm for giardiasis and amebiasis.
3. List one effective therapy for nematodes and select the drugs of choice for strongyloidiasis and tapeworms.
4. List three major reasons why travelers are infected with malaria.
5. Describe the presenting signs and symptoms of malaria.
6. List some specific toxicities of mefloquine.
7. Identify the monitoring parameters for quinidine gluconate in severe malaria.
8. Define the major complications of falciparum malaria.
9. Discuss the cardiovascular complications of chronic South American trypanosomiasis.
10. Describe the steps to take to eradicate lice infestation and scabies.
KEY CONCEPTS
![]() For treatment of giardiasis (or as empirical treatment), metronidazole 250 mg three times daily for 7 days or tinidazole 2 g as a single dose is recommended.
For treatment of giardiasis (or as empirical treatment), metronidazole 250 mg three times daily for 7 days or tinidazole 2 g as a single dose is recommended.
![]() Diagnostic tests for amebiasis include stool for ova, antigen detection, or polymerase chain reaction (PCR) testing.
Diagnostic tests for amebiasis include stool for ova, antigen detection, or polymerase chain reaction (PCR) testing.
![]() The drug of choice for nematode infestations (hookworm, enterobiasis, and ascariasis) is mebendazole, while ivermectin is indicated for strongyloidiasis and praziquantel is indicated for tapeworms.
The drug of choice for nematode infestations (hookworm, enterobiasis, and ascariasis) is mebendazole, while ivermectin is indicated for strongyloidiasis and praziquantel is indicated for tapeworms.
![]() The primary reasons why travelers are infected with malaria are failure to take chemotherapy, inappropriate chemotherapy, and delay in seeking medical care.
The primary reasons why travelers are infected with malaria are failure to take chemotherapy, inappropriate chemotherapy, and delay in seeking medical care.
![]() Falciparum malaria must be considered a life-threatening medical emergency.
Falciparum malaria must be considered a life-threatening medical emergency.
![]() Treatment of serious malarial infection requires admission to an acute care service, IV administration of quinidine gluconate, and symptomatic support.
Treatment of serious malarial infection requires admission to an acute care service, IV administration of quinidine gluconate, and symptomatic support.
![]() Complications of falciparum malaria include hypoglycemia, acute renal failure, pulmonary edema, seizure, and coma.
Complications of falciparum malaria include hypoglycemia, acute renal failure, pulmonary edema, seizure, and coma.
![]() The chronic presentation of American trypanosomiasis includes cardiovascular, GI, and CNS manifestations.
The chronic presentation of American trypanosomiasis includes cardiovascular, GI, and CNS manifestations.
![]() Lice infestation should be treated with 1% permethrin followed by treatment of immediate family members and sexual partners. Bedding and clothes should be sterilized by washing in the hot cycle of the washing machine.
Lice infestation should be treated with 1% permethrin followed by treatment of immediate family members and sexual partners. Bedding and clothes should be sterilized by washing in the hot cycle of the washing machine.
![]() The diagnosis of scabies is made by obtaining skin scrapings and detecting the mite in a wet mount. Topical therapy is 5% permethrin.
The diagnosis of scabies is made by obtaining skin scrapings and detecting the mite in a wet mount. Topical therapy is 5% permethrin.
Parasitic medicine is an ever changing field. The increased desire of large segments of the U.S. population to travel to Asia, Africa, and other parts of the world can expose them to parasitic infections that are endemic in those areas. The influx of refugees and new immigrant populations from Asia and other parts of the world have brought new parasitic infections to our shores. Migrant farm workers who work and live in substandard hygienic conditions, the large and growing Central and South American immigrant population, and the presence of immunosuppressed populations (e.g., those with the AIDS and transplant patients) represent other significant sources of parasitic infections in the United States.1–10 Clearly, there is a need for health professionals in the United States to be familiar with the pathophysiology and treatment of parasitic diseases.
Defined below are some terms that are frequently used when discussing parasitic diseases.9 Symbiosis is defined as “living together,” when two species are dependent on each other for food and protection. The term commensalism, from the Latin translation of “eating at the same table,” implies a mutual association in which both organisms may benefit, or at least one benefits but does no harm to the other. In contrast, parasitism, although resembling symbiosis in one aspect (i.e., it is also an intimate relationship between two species), does not represent a mutually beneficial association. One species (the host) does not benefit from the relationship, and in fact the relationship may be detrimental to its very survival. Parasites have made morphologic, biochemical, reproductive, and defensive adaptations over time. These adaptations have increased the ability of parasites to survive host defenses and have allowed them to utilize the host’s biochemical systems to synthesize necessary cellular components. Beef and pork tapeworms (cestodes) possess highly developed reproductive systems which allow them to transfer easily to new hosts. Because of the lack of digestive systems cestodes are completely host-dependent for all nutrients. Cestodes (tapeworms) (Taenia saginata and T. solium) use specialized suckers which enable them to obtain blood and vital nutrients from their host. Entamoeba histolytica, the causative agent for amebiasis, once it has gained access to the human colon or large intestine is able to invade and utilize its specialized proteolytic enzyme to penetrate and erode the GI mucosa. E. histolytica is also able to survive in adverse conditions when it leaves the host by walling itself off and forming cysts; this protects the parasite from environmental conditions until it is ready to infect the next host.
Although acquired immunity to some parasitic diseases may lower the level of infection, absolute immunity as seen in bacterial and viral infections is seldom seen in parasitic diseases. Since parasitic infections produce a wide variety of antigens because of the many life cycle phases, it is more difficult to identify a constant antigenic protein against which specific antibodies are protective. However, malaria remains a likely candidate for a vaccine and there are ongoing studies to develop one.
Space constraints do not allow detailed discussions of the world of parasites, and clinicians and students are directed to some excellent resources for further details on parasites and parasitic diseases.9,11Discussion in this chapter will include those parasitic diseases that are more likely to be seen in the United States and will include GI parasites (primarily giardiasis and amebiasis), protozoan infections (malaria and South American trypanosomiasis), some common helminthic diseases (specifically those caused by nematodes and cestodes), and ectoparasites (lice and scabies).
GIARDIASIS
EPIDEMIOLOGY AND ETIOLOGY
Giardia lamblia (also known as G. intestinalis or G. duodenalis), an enteric protozoan, is the most common intestinal parasite responsible for diarrheal syndromes throughout the world. Giardia is the most frequently identified intestinal parasites in the United States, with a prevalence rate of 5% to 15% in some areas. G. lamblia has been identified as the first enteric pathogen seen in children in developing countries, with prevalence rates between 15% and 30%.
Patient Encounter 1: Giardiasis
MK is a 15-year-old high school student who had traveled to Mexico as part of a school group to practice his Spanish language skills. While in Mexico, he was careful not to drink any local water and only consumed warm or heated food and soda. He is seen in the travel clinic with complaints of some “explosive” crampy diarrhea and has had constipation alternating with diarrhea for the last 2 weeks. MK indicates that his stools have been foul smelling.
Are his symptoms characteristic of giardiasis?
How would you differentiate giardiasis from possible
Escherichia coli–induced diarrhea?
There are two stages in the life cycle of G. lamblia: the trophozoite and the cyst. G. lamblia is found in the small intestine, the gallbladder, and in biliary drainage. The distribution of giardiasis is worldwide with children being more susceptible than adults.
PATHOPHYSIOLOGY
Giardiasis is caused by ingestion of G. lamblia cysts in fecally-contaminated water or food.9–15 The protozoan excysts in the low gastric pH to release the trophozoite. Colonization and multiplication of the trophozoite lead to mucosal invasion, localized edema, and flattening of the villi, resulting in malabsorption states in the host. Achlorhydria, hypogammaglobulinemia, or deficiency in secretory immunoglobulin A (IgA) predispose to giardiasis. Individuals with HIV infection and AIDS may have higher carriage rates than the general population. Some patients may develop lactose intolerance after chronic giardiasis.
CLINICAL PRESENTATION AND DIAGNOSIS
Pharmacologic Therapy
![]() All symptomatic adults and children over the age of 8 years with giardiasis should be treated with metronidazole 250 mg three times daily for 7 days, or tinidazole 2 g as a single dose, or nitazoxanide (Alina) 500 mg twice daily for 3 days.12,16 The pediatric dose of metronidazole is 15 mg/kg/day three times daily for 7 days. Alternative drugs include furazolidone 100 mg four times daily or paromomycin 25 to 35 mg/kg/day in divided doses daily for 7 days. Paromomycin may be used in pregnancy instead of metronidazole. Pediatric patients can also be treated with suspensions of either furazolidone 6 mg/kg/day in four divided doses for 7 days.
All symptomatic adults and children over the age of 8 years with giardiasis should be treated with metronidazole 250 mg three times daily for 7 days, or tinidazole 2 g as a single dose, or nitazoxanide (Alina) 500 mg twice daily for 3 days.12,16 The pediatric dose of metronidazole is 15 mg/kg/day three times daily for 7 days. Alternative drugs include furazolidone 100 mg four times daily or paromomycin 25 to 35 mg/kg/day in divided doses daily for 7 days. Paromomycin may be used in pregnancy instead of metronidazole. Pediatric patients can also be treated with suspensions of either furazolidone 6 mg/kg/day in four divided doses for 7 days.
Clinical Presentation and Diagnosis of Giardiasis
Acute Onset
• Diarrhea, cramp-like abdominal pain, bloating, and flatulence
• Malaise, anorexia, nausea, and belching
Chronic Symptoms
• Diarrhea: Foul-smelling, copious, light-colored and greasy stools
• Weight loss, steatorrhea, and vitamin B12 and fat-soluble vitamin deficiencies
• Constipation alternating with diarrhea
Diagnosis
• Diagnosis is made by examination of fresh stool or a preserved specimen during acute diarrheal phase
• Fresh stool may show trophozoites while preserved specimens yield cysts. (Note: stool for ova may show the presence of other parasites [e.g., Cryptosporidium parvum, E. histolytica, or E. hartmanni]; multiple stool samples may be needed.)
• Even though stool examination for ova and parasites has remained the major means of diagnosis, other diagnostic tests include enzyme-linked immunosorbent assay (ELISA), which is considered to be between 85% and 98% sensitive and almost 100% specific (ProSpec T, Giardia Microplate Assay, Remel, Lenexa, KS).
Quinacrine 100 mg three times in adults or 5 mg/kg/day in pediatric patients for 5 to 7 days, is available from a specialized pharmacy (e.g., Ponorama Compounding Pharmacy).12
Patient Care and Monitoring: Giardiasis
• Metronidazole produces cure rates between 85% and 95%.
• Diarrhea will cease within a few days, although in some patients it may take 1 to 2 weeks.
• Cyst excretion will cease within days.
• Intestinal dysfunction (manifested as increased transit time) and radiologic changes primarily due to chronic infection may take months to resolve.
• Patients who fail therapy with metronidazole should receive a second course with either metronidazole or an alternative agent; nitazoxanide has been shown to be effective in resistant giardiasis.
OUTCOME EVALUATION
Patients with symptomatic giardiasis and positive stool samples or positive enzyme-linked immunosorbent assay (ELISA) tests should be treated with metronidazole for 7 days. Patients who fail initial therapy with metronidazole should receive a second course of therapy. Pregnant patients can receive paromomycin 25 to 35 mg/kg/day in divided doses for 7 days. Giardiasis can be prevented by good hygiene and by using caution in food and drink consumption.
AMEBIASIS
EPIDEMIOLOGY AND ETIOLOGY
Amebiasis remains one of the most important parasitic diseases because of its worldwide distribution and serious GI manifestations. The major causative agent in amebiasis is E. histolytica, which invades the colon and must be differentiated from E. dispar, which is associated with an asymptomatic carrier state and is considered nonpathogenic.17–20 Invasive amebiasis is almost exclusively the result of ingesting E. histolytica cysts found in fecally-contaminated food or water. Approximately 50 million cases of invasive disease result each year worldwide, leading to an excess of 100,000 deaths. In the general population, the highest incidence is found in institutionalized mentally retarded patients, sexually active homosexuals, AIDS patients, the Native American population, and new immigrants from endemic areas (e.g., Mexico, South and Southeast Asia, West and South Africa, and portions of Central and South America).
PATHOPHYSIOLOGY
E. histolytica invades mucosal cells of colonic epithelium, producing the classic flask-shaped ulcer in the submucosa. The trophozoite toxin has a cytocidal effect on cells. If the trophozoite gets into the portal circulation, it will be carried to the liver, where it produces abscess and periportal fibrosis. Liver abscesses are more common in men than women and are rarely seen in children. Amebic ulcerations can affect the perineum and genitalia, and abscesses may occur in the lung and brain.
Erosion of liver abscesses can result in peritonitis. Liver abscesses that are located in the right lobe can spread to the lungs and pleura. Pericardial infection, although rare, may be associated with extension of the amebic abscesses from the liver.21–23
CLINICAL PRESENTATION AND DIAGNOSIS
Pharmacologic Therapy
Metronidazole (Flagyl), dehydroemetine, and chloroquine (Aralen) are tissue-acting agents, and iodoquinol (Yodoxin), diloxanide furoate (Furamide), and paromomycin (Humatin) are luminal amebicides. A systemic or tissue-acting agent may be so well absorbed that the amounts of the drug remaining in the bowel may be insufficient to have luminal or local effects. A luminally-active agent, on the other hand, may not attain effective enough levels in the tissue to be efficacious. Asymptomatic cyst passers (identified by stool examinations, and who may develop invasive disease) and patients with mild intestinal amebiasis should receive a luminal agent: paromomycin 25 to 35 mg/kg/day three times daily for 7 days, or iodoquinol 650 mg three times daily for 20 days, or diloxanide furoate 500 mg three times daily for 10 days. These regimens have cure rates of between 84% and 96%. Dilox anide furoate is available from Ponorama Compounding Pharmacy (6744 Balboa Blvd., Lake Balboa, CA 91406; [800] 247-9767).12The pediatric dose of paromomycin is the same as that used in adults, whereas the pediatric dose of iodoquinol is 30 to 40 mg/kg (maximum: 2 g) per day in three doses for 20 days, and the pediatric dose of diloxanide furoate is 20 mg/kg/day in three doses for 10 days. Paromomycin is the preferred agent in pregnant patients.12
Clinical Presentation and Diagnosis of Amebiasis
Review of the patient’s history should include: recent travel, type of foods ingested (e.g., salads or unpeeled fruit), the nature of water and fluid consumed, and description of any symptoms of friends or relatives who ate the same food.
Intestinal Disease
• Vague abdominal discomfort
• Symptoms may range from malaise to severe abdominal cramps, flatulence, and nonbloody or bloody diarrhea (heme-positive in 100% of cases) with mucus
• May have low-grade fever, but this may be absent in many patients
• Eosinophilia is usually absent, although mild leukocytosis is not unusual
Note: Fecal screening may show other intestinal parasites, including Cryptosporidium spp., Balantidium coli, Dientamoeba fragilis, Isospora belli, G. lamblia, or Blastocystis hominis.
Amebic Liver Abscess
• May present with high fever with significant leukocytosis with left shift, anemia, elevated alanine aminotransferase, and dull abdominal pain on palpation
• Physical findings: Right upper quadrant pain, hepatomegaly, and liver tenderness, with referred pain to the left or right shoulder (Note: Erosion of liver abscesses may present as peritonitis.)
![]() Diagnosis
Diagnosis
• Intestinal amebiasis is diagnosed by demonstrating E. histolytica cysts or trophozoites (may contain ingested erythrocytes) in fresh stool or from a specimen obtained by sigmoidoscopy.
• Microscopy may not differentiate between the pathogenic E. histolytica and the nonpathogenic E. dispar or E. moshkovskii in stools.
• Sensitive techniques are available to detect E. histolytica in stool: antigen detection, antibody test (ELISA) and PCR.
• Endoscopy with scrapings or biopsy and stained slides (iron hematoxylin or trichrome) may provide more definitive diagnosis of amebiasis.
• Diagnosis for liver abscess includes serology and liver scans (using isotopes by ultrasound or CT) or MRI; however, none of these are specific for liver abscess. In rare instances, needle aspiration of hepatic abscess may be attempted using ultrasound guidance.
Patients with severe intestinal disease or liver abscess should receive metronidazole 750 mg three times daily for 10 days, followed by the luminal agents indicated above. The pediatric dose of metronidazole is 50 mg/kg/day in divided doses, which should be followed by a luminal agent. An alternative regimen of metronidazole is 2.4 g/day for 2 days in combination with the luminal agent.20,21 Tinidazole (Tindamax, recently introduced in the U.S. market) administered in a dose of 2 g daily for 3 days (pediatric dose: 50 mg/kg for 3 days) is an alternative to metronidazole. If there is no prompt response to metronidazole or aspiration of the abscess, an antibiotic regimen should be added. Patients who cannot tolerate oral doses of metronidazole should receive an equivalent dose IV.
Patient Encounter 2: Amebiasis
WR is a 37-year-old native of India and a permanent resident in the United States who has recently returned from a trip to Calcutta where he was visiting a relative. He presents in the emergency department with complaints of a 3-week history of sharp, crampy, and postprandial abdominal pain. The pain is more intense over the right lower quadrant and associated with watery nonbloody diarrhea and tenesmus.
What specific findings in this patient suggest that he may have giardiasis or amebiasis?
What other information do you need to confirm a diagnosis of amebiasis?
What is the major complication of amebiasis?
Patient Care and Monitoring: Amebiasis
1. Follow-up in patients with amebiasis should include repeat stools (1–3), colonoscopy (in colitis) or CT (in liver abscess) between days 5 and 7, at the end of the course of therapy, and a month after the end of therapy.
2. Most patients with either intestinal amebiasis or colitis will respond in 3 to 5 days with amelioration of symptoms.
3. Those with liver abscess may take up to 7 days before there will be decreases in pain and fever. In liver abscess, patients not responding by the fifth day may require aspiration of the abscesses or exploratory laparotomy.
4. Serial liver scans have demonstrated that healing of liver abscesses take from 4 to 8 months following adequate therapy.
Preventive Measures
• Travelers and tourists visiting endemic areas should avoid local tap water, ice, salads, and unpeeled fruits. Boiled water is safe.
• Water can be disinfected by the use of iodine 2% (5 drops/L) or chlorine 6% (laundry bleach: 4 drops/L) or use of a commercial water purifier, such as Portable Aqua tablets (Wisconsin Pharmaceutical).
OUTCOME EVALUATION
Follow-up in patients with amebiasis should include repeat stool examinations, serology, colonoscopy (in colitis) or CT on day 7, at the end of therapy, and a month after the end of therapy. Serial liver scans have demonstrated healing of liver abscesses over 4 to 8 months after adequate therapy.20,21
HELMINTHIC DISEASES
Helminthic infections include three groups of organisms: roundworms or nematodes, flukes (trematodes), and tapeworms (cestodes). Because of space constraints, only brief descriptions of some of the helminthic infections most commonly seen in North America and their treatments will be provided here. Although helminthic infections may not produce clinical manifestations, they can cause significant pathology. One factor that determines the pathogenicity of helminthic infections is their population density; a high-density population (“worm burden”) results in predictable disease presentation. In the United States, these infections are reported most frequently in recent immigrants from Southeast Asia, the Caribbean, Mexico, and Central America.5,6 Populations at risk include institutionalized patients (both young and elderly), preschool children in daycare centers, residents of Native American reservations, and homosexuals.17,24 Certain conditions and drugs (anesthesia and corticosteroids) can cause atypical localization of worms. Immunocompromised hosts can be overwhelmed by some helminthic infections, such as Strongyloides stercoralis.
NEMATODES
Hookworm Disease
Hookworm infection is caused by Ancylostoma duodenale or Necator americanus. N. americanus is found in the southeastern United States.24–26 Infective larvae enter the host in contaminated food or water, or penetrate the skin and migrate to the small intestine. The adult worm attaches to GI mucosa and causes injury by lytic destruction of the tissue. Over a period of time, the adult worm can cause anemia and hypoproteinemia in the host.27,28
Treatment
![]() The drug of choice is mebendazole (Vermox), which is also active against ascariasis, enterobiasis, trichuriasis, and hookworm.12 The adult and pediatric (age greater than 2 years) oral dose of mebendazole for hookworm is 100 mg twice daily for 3 days. An alternative agent that can be used in both pediatric and adult patients is albendazole (Zentel), 400 mg as a single oral dose. Diagnosis is by detection of eggs or larvae in stool. Stool examination for eggs and the larvae should be repeated in 2 weeks and the patient retreated if necessary.
The drug of choice is mebendazole (Vermox), which is also active against ascariasis, enterobiasis, trichuriasis, and hookworm.12 The adult and pediatric (age greater than 2 years) oral dose of mebendazole for hookworm is 100 mg twice daily for 3 days. An alternative agent that can be used in both pediatric and adult patients is albendazole (Zentel), 400 mg as a single oral dose. Diagnosis is by detection of eggs or larvae in stool. Stool examination for eggs and the larvae should be repeated in 2 weeks and the patient retreated if necessary.
ASCARIASIS
The causative agent in ascariasis is the giant roundworm Ascaris lumbricoides, which is found worldwide and is responsible about 4 million infections in the United States (it primarily affects residents of the Appalachian mountain range and the Gulf Coast states).29–31 Migration of the worm into the lungs usually produces pneumonitis, fever, cough, eosinophilia, and pulmonary infiltrates. Ascaris infection can also cause abdominal discomfort, intestinal obstruction, and appendicitis. Diagnosis is made by detection of the characteristic eggs in the stool or passed worms.
Treatment
In both adults and pediatric patients older than 2 years of age, mebendazole 100 mg orally twice daily for 3 days is the treatment to use. An alternative agent is pyrantel pamoate (Antiminth).12 The stool should be checked within 2 weeks and the patient retreated when warranted.
ENTEROBIASIS
Enterobiasis, or pinworm infection, is caused by Enterobius vermicularis. It is the most widely distributed helminthic infection in the world.24,32 There are approximately 42 million cases in the United States, primarily affecting children. The most common manifestation of the infection is cutaneous irritation in the perianal region, resulting from the migrating female or the presence of eggs. The intense pruritus may lead to dermatitis and secondary bacterial infections. Diagnosis is made by the use of a perianal swab and cellophane tape sampling, which will aid in egg identification.
Treatment
The three agents that are administered for enterobiasis include pyrantel pamoate, mebendazole, and albendazole. The oral dose of pyrantel pamoate is 11 mg/kg (maximum: 1 g) as a single dose that can be repeated in 2 weeks. The oral dose of mebendazole for both adults and children older than 2 years of age is 100 mg as a single dose. This may be repeated in 2 weeks.12 Following treatment, to eradicate the eggs, all bedding and underclothing should be sterilized by steaming or washing in the hot cycle of the washing machine.
STRONGYLOIDIASIS
Strongyloidiasis is caused by Strongyloides stercoralis, which has a worldwide distribution and is predominantly prevalent in South America (Brazil and Columbia) and in Southeast Asia.33–38 Strongyloidiasis is primarily seen among institutionalized populations (those in mental hospitals and children’s hospitals) and immunocompromised individuals (those with HIV infection, AIDS, and patients with hematologic malignancies).33,35 The worm is usually found in the upper intestine where the eggs are deposited and hatch to form the rhabditiform larvae. The rhabditiform larva (male and female) migrate to the bowel where they may be excreted in the feces. If excreted in the feces, the larva can evolve into either one of two forms after copulation: a free-living noninfectious rhabditiform larvae, or an infectious filariform larvae. The filariform larva can penetrate host skin and migrate to the lungs and produce progeny, a process called autoinfection. This can result in hyperinfection (i.e., an increased number of larva in the intestine, lungs, and other internal organs), especially in an immunocompromised host.
Patients with acute infection may develop a localized pruritic rash, but heavy infestations can produce eosinophilia (10–15%), diarrhea, abdominal pain, and intestinal obstruction. Administration of corticosteroids or other immunosuppressive drugs to an infected individual can result in hyperinfections and disseminated strongyloidiasis.33,35 Diagnosis of strongyloidiasis is made by identification of the rhabditiform larva in stool, sputum, or duodenal fluid, or from small bowel biopsy specimens or via antigen testing (ELISA essay). Multiple stool and other samples may need to be checked, both for diagnosis and to ensure eradication of the larva in patients after treatment.
Treatment
![]() The drug of choice for strongyloidiasis is oral ivermectin 200 mcg/kg/day for 2 days, while albendazole 400 mg twice daily is given for 7 days as an alternative.12,39 With hyperinfection or disseminated strongyloidiasis, immunosuppressive drugs should be discontinued and treatment should be initiated with ivermectin 200 mcg/kg/day until all symptoms are resolved. Patients should be tested periodically to ensure the elimination of the larva. Individuals from an endemic area who are candidates for organ transplantation should be screened for S. stercoralis.
The drug of choice for strongyloidiasis is oral ivermectin 200 mcg/kg/day for 2 days, while albendazole 400 mg twice daily is given for 7 days as an alternative.12,39 With hyperinfection or disseminated strongyloidiasis, immunosuppressive drugs should be discontinued and treatment should be initiated with ivermectin 200 mcg/kg/day until all symptoms are resolved. Patients should be tested periodically to ensure the elimination of the larva. Individuals from an endemic area who are candidates for organ transplantation should be screened for S. stercoralis.
CESTODIASIS
Cestodiasis (tapeworm infection) is caused by species of the phylum Platyhelminthes (flatworms), and include among others the pork tapeworm (Taenia solium) and the beef tapeworm (T. saginata).9,40 The tapeworm attaches itself to the mucosal wall of the upper jejunum by the scolex (mouth parts), and by two to four cup-shaped suckers and a structure called a rostellum, which may have hooks in some species. Since the parasite lacks a digestive system it obtains all nutrients directly from the host. The scolex, proglottids (segments), and eggs are specific for each species and used for identification of tapeworms. Tapeworm infections are caused by ingestion of poorly cooked meat which contains the larva or cysticerci. Cysticerci, when released from the contaminated meat by host digestive juices, mature in the host jejunum. Cystericercosis is a systemic disease caused by the larva of T. solium (oncosphere or hexacanth), and is usually acquired by ingestion of eggs in contaminated food or by autoinfection.41–45 The larvae can penetrate the bowel and migrate through the bloodstream to infect different organs including the CNS (neurocysticercosis). Diagnosis of both T. saginata and T. solium is accomplished by recovery of the gravid proglottids and the scolex in the stool.
Treatment
![]() Tapeworm infections (T. saginata and T. solium) are treated with praziquantel 5 to 10 mg/kg as a single dose (use the same dose for adults and pediatric patients).12 The treatment for cysticercosis and neurocysticercosis may include surgery, anticonvulsants (neurocysticercosis can cause seizures), and anthelmintic therapy. The anthelmintic therapy of choice is albendazole 400 mg twice daily for 8 to 30 days.45,46 The pediatric dose of albendazole is 15 mg/kg (maximum: 800 mg) in two divided doses for 8 to 30 days. The doses for both adults and pediatric subjects can be repeated if necessary. Praziquantel is an alternative therapy.12
Tapeworm infections (T. saginata and T. solium) are treated with praziquantel 5 to 10 mg/kg as a single dose (use the same dose for adults and pediatric patients).12 The treatment for cysticercosis and neurocysticercosis may include surgery, anticonvulsants (neurocysticercosis can cause seizures), and anthelmintic therapy. The anthelmintic therapy of choice is albendazole 400 mg twice daily for 8 to 30 days.45,46 The pediatric dose of albendazole is 15 mg/kg (maximum: 800 mg) in two divided doses for 8 to 30 days. The doses for both adults and pediatric subjects can be repeated if necessary. Praziquantel is an alternative therapy.12
OUTCOME EVALUATION
Morbidity and disease due to helminthic infections is related to the intensity of infection. The major adverse effects of helminthic infections are malnutrition, fatigue, and diminished work capacity. Unlike other helminthic infections, strongyloidiasis can cause autoinfection, and in the presence of immunosuppression, it can cause CNS and disseminated infections which have high mortality.33
The most serious complication of cysticercosis is neurocysticercosis that can cause strokes and seizures.45 Treatment of neurocysticercosis with anthelmintic treatment remains controversial.
MALARIA
Malaria is one of the most devastating parasitic diseases, affecting a population in excess of 500 million and causing between 700,000 and 2.7 million deaths a year worldwide.3,5,7 In the year 2000, approximately 27 million U.S. travelers visited countries where malaria is endemic. In 2002, the Centers for Disease Control and Prevention indicated that there were 1,337 cases of malaria, of which 849 were in U.S. civilians, 33 in U.S. military personnel, and the rest in foreign civilians.5 There were eight fatalities, all due to Plasmodium falciparum. ![]() The primary reasons for morbidity and death in malaria are failure to take recommended chemoprophylaxis, inappropriate chemoprophylaxis, delay in seeking medical care or in initiating therapy promptly, and misdiagnosis. Evaluation of a patient should include specific travel history, details of chemoprophylaxis, and physical findings (e.g., splenomegaly).
The primary reasons for morbidity and death in malaria are failure to take recommended chemoprophylaxis, inappropriate chemoprophylaxis, delay in seeking medical care or in initiating therapy promptly, and misdiagnosis. Evaluation of a patient should include specific travel history, details of chemoprophylaxis, and physical findings (e.g., splenomegaly).
Malaria is transmitted by the bites of the Anopheles mosquitoes which introduce into the bloodstream one of four species of sporozoites of the plasmodia (Plasmodium falciparum, P. ovale, P. vivax, or P. malariae).47–56 Initial symptoms of malaria are nonspecific and may resemble influenza and include: chills, headache, fatigue, muscle pain, rigors, and nausea. The onset of the symptoms is between 1 and 3 weeks following exposure. Fever may appear 2 to 3 days after initial symptoms and may follow a pattern and occur every 2 or 3 days (P. vivax, P. ovale, and P. malariae). Fever with P. falciparum can be erratic and may not follow specific patterns. It is not unusual for patients to have concomitant infections with P. vivax and P. falciparum. Falciparum malaria must always be regarded as a life-threatening medical emergency.
EPIDEMIOLOGY AND ETIOLOGY
The distribution of the various species of malaria is not well defined but P. vivax is reported to be prevalent in the Indian subcontinent, Central America, North Africa, and the Middle East, whereas P. falciparum is predominantly in Africa (including sub-Saharan Africa), both East and West Africa, Haiti, the Dominican Republic, the Amazon region of South America, Southeast Asia, and New Guinea.5,48,51Most P. ovale infections occur in Africa, while the distribution of P. malariae is worldwide.7 Most infections in the United States are reported in American travelers, recent immigrants, or immigrants who have visited friends and family in an endemic area.4,7 Placental transmission and blood transfusions are also sources of malaria.
Within minutes after the bite of the Anopheles mosquito, the sporozoites invade hepatocytes in the liver and begin an asexual phase called schizonts (exoerythrocytic stage or schizogony). The patient may be asymptomatic during this period. After a lapse of between 5 and 15 days (depending on the species), schizonts rupture to release daughter cells (merozoites) into the blood, which then invade erythrocytes. In erythrocytes the merozoites undergo a number of sequential forms: a ring form, trophozoite, schizont, and merozoite, which then invade new erythrocytes. This asexual phase is about 48 hours for P. falciparum, P. vivax, and P. ovale, and 72 hours for P. malariae. Subsequently, the merozoites develop into gametocytes and undergo a sexual phase (sporogony) in the Anopheles mosquito. In the mosquito, the gametocytes undergo a number of stages: zygote, ookinete, and oocyst, and finally transform into sporozoites in the salivary glands where it is again able to infect the next host. Unlike P. falciparum and P. malariae, which only remain in the liver for about 3 weeks before invading erythrocytes, P. ovale and P. vivax can remain in the liver for extended periods in a latent stage (as hypnozoites); this can result in the recurrence of the infection after weeks or months. Primaquine therapy is necessary to eradicate this stage of the infection.
Patient Encounter 3, Part 1: Malaria
TW is a 27-year-old male who had returned from Bamako, Mali in West Africa, after visiting his college classmate who was in the Peace Corps. While there, he accompanied his friend on a river trip to visit a number of villages. He indicates that he took steps to minimize mosquito bites and had slept under a mosquito net. He was well since returning from Africa until the previous day, when he had a temperature as high as 39°C (102.2°F), with anorexia, headache, chills, sweats, myalgias, and abdominal pain. He took a few doses of ibuprofen but his fever came back after a few hours and he now presents in the emergency department with chills, high fever (greater than 39.8°C) (greater than 103.6°F), headache, abdominal pain, nausea, stiffness of the neck, and back pain.
Are the symptoms in this patient consistent with malaria?
What places this patient at risk for malaria?
What additional information do you need to develop a therapeutic plan for this patient?
PATHOPHYSIOLOGY
The clinical presentation of malaria can be quite variable. Normally, the appearance of a prodrome with headache, abdominal pain, fatigue, fever, and chills, which coincides with the erythrocytic phase of malaria occurs frequently between 10 and 21 days after being exposed.48,51 This phase causes extensive hemolysis, which results in anemia and splenomegaly. The most serious complications are caused by P. falciparum infections. Infants and children under the age of 5 years and nonimmune pregnant women are at high risk for severe complications with falciparum infections.52–58 The complications associated with falciparum malaria are related to two unique features of P. falciparum: (a) its ability to produce high parasitism (up to 80%) of red cells of all ages; and (b) the propensity to be sequestered in postcapillary venules of critical organs such as brain, liver, heart, lungs, and kidneys.53,54 It has been postulated that tissue hypoxia from anemia, together with P. falciparum–parasitized red blood cell adherence to endothelial cells in capillaries, contribute to severe ischemia and metabolic derangements. P. malariae is implicated in immune-mediated glomerulonephritis and nephrotic syndrome.
CLINICAL PRESENTATION AND DIAGNOSIS
Recent innovations for detecting malaria include DNA or RNA probes by polymerase chain reaction (PCR).59 These, however, are not widely available for clinical use. A rapid dip-stick test (ParaSight F, Becton-Dickinson, Cockeyville, MD) reportedly has a sensitivity of 88% and a specificity of 97%, which is comparable to microscopy. However, ParaSight F can give false-positive results with rheumatoid factor; thus microscopy remains the optimal test.
Clinical Presentation and Diagnosis of Malaria
Initial Presentation
Include a careful travel history of patient and physical findings (e.g., splenomegaly) and details of antimalarial chemoprophylaxis, when obtainable.
Erythrocytic Phase
1. Prodrome: Headache, anorexia, malaise, fatigue, and myalgia
2. Nonspecific complaints include: abdominal pain, diarrhea, chest pain, and arthralgia
3. Paroxysm: High fever, chills, and rigor
4. Cold phase: Severe pallor, cyanosis of the lips and nail beds
5. Hot phase: Fever between 40.5°C (104.9°F) and 41°C (105.8°F) (seen more frequently with P. falciparum)
6. Sweating phase: Follows the hot phase by 2 to 6 hours
7. When fever resolves, it is followed by marked fatigue and drowsiness, warm dry skin, tachycardia, cough, headache, nausea, vomiting, abdominal pain, diarrhea and delirium, anemia, and splenomegaly
![]() P. falciparum malaria is a life-threatening emergency. Complications include hypoglycemia, acute renal failure, pulmonary edema, severe anemia (high parasitism), thrombocytopenia, heart failure, cerebral congestion, seizures, coma, and adult respiratory distress syndrome.
P. falciparum malaria is a life-threatening emergency. Complications include hypoglycemia, acute renal failure, pulmonary edema, severe anemia (high parasitism), thrombocytopenia, heart failure, cerebral congestion, seizures, coma, and adult respiratory distress syndrome.
Diagnostic Procedures for Malaria
1. To ensure a positive diagnosis, blood smears (both thick and thin films) should be obtained every 12 to 24 hours for three consecutive days.
2. The presence of parasites in the blood 3 to 5 days after initiation of therapy suggests resistance to the drug regimen.
TREATMENT
The primary goal in the management of malaria is the rapid identification of the Plasmodium species by blood smears (both thick and thin smears repeated every 12 hours for 3 days). Antimalarial therapy should be initiated promptly to eradicate the infection within 48 to 72 hours and avoid complications such as hypoglycemia, pulmonary edema, and renal failure.53,54
PHARMACOLOGIC THERAPY
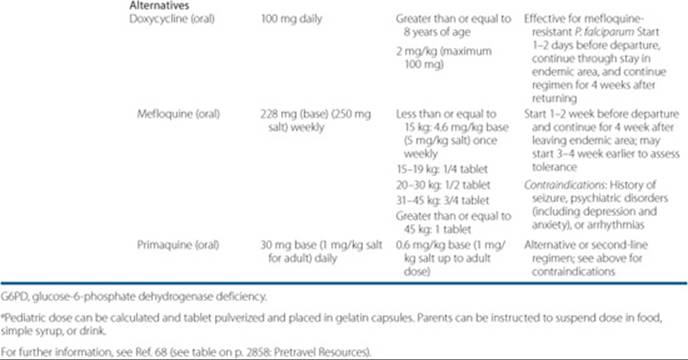
The chemoprophylaxis regimen for malaria is outlined in Table 78–1.12
Chemotherapy for Malarial Infection
In an uncomplicated attack of malaria (for all plasmodia except chloroquine-resistant P. falciparum and P. vivax), the recommended oral regimen is chloroquine 600 mg (base) initially, followed by 300 mg (base) 6 hours later, and then 300 mg (base) daily for 2 days.12 ![]() In severe illness or falciparum malaria, patients should be admitted to an acute care unit and quinidine gluconate 10 mg salt/kg as a loading dose (maximum 600 mg) in 250 mL normal saline should be administered IV slowly over 1 to 2 hours. This should be followed by continuous infusion of 0.02 mg/kg/min of quinidine for at least 24 hours until oral therapy can be started. In patients who have received either quinine or mefloquine, the loading dose of quinidine should be omitted. Oral quinine salt (650 mg every 8 hours) plus doxycycline 100 mg twice daily should follow the IV dose of quinidine to complete 7 days of therapy.53,54 The pediatric dose of IV quinidine gluconate is the same as the dose for adults. The pediatric dose of oral quinine is 25 mg/kg/day in three divided doses, while the dose of doxycycline (children greater than 8-year-old) is 4 mg/kg in two divided doses for 7 days. An alternative to doxycycline is clindamycin 900 mg (20 mg/kg/day) three times daily for 3 days. The pediatric dose of clindamycin is the same as in adults. (In patients who cannot tolerate quinidine or quinidine is not readily available, IV artesunate 2.4 mg/kg/dose × 3 days, at 0,12, 24, 48, and 72 hours may be used, followed by oral therapy. Artesunate is available from CDC under an investigational new drug application. Pediatric dose of artesunate is same as in adults. Oral therapy may include atovaquone/proguanil, doxycycline, mefloquine or clindamycin.)12
In severe illness or falciparum malaria, patients should be admitted to an acute care unit and quinidine gluconate 10 mg salt/kg as a loading dose (maximum 600 mg) in 250 mL normal saline should be administered IV slowly over 1 to 2 hours. This should be followed by continuous infusion of 0.02 mg/kg/min of quinidine for at least 24 hours until oral therapy can be started. In patients who have received either quinine or mefloquine, the loading dose of quinidine should be omitted. Oral quinine salt (650 mg every 8 hours) plus doxycycline 100 mg twice daily should follow the IV dose of quinidine to complete 7 days of therapy.53,54 The pediatric dose of IV quinidine gluconate is the same as the dose for adults. The pediatric dose of oral quinine is 25 mg/kg/day in three divided doses, while the dose of doxycycline (children greater than 8-year-old) is 4 mg/kg in two divided doses for 7 days. An alternative to doxycycline is clindamycin 900 mg (20 mg/kg/day) three times daily for 3 days. The pediatric dose of clindamycin is the same as in adults. (In patients who cannot tolerate quinidine or quinidine is not readily available, IV artesunate 2.4 mg/kg/dose × 3 days, at 0,12, 24, 48, and 72 hours may be used, followed by oral therapy. Artesunate is available from CDC under an investigational new drug application. Pediatric dose of artesunate is same as in adults. Oral therapy may include atovaquone/proguanil, doxycycline, mefloquine or clindamycin.)12
In P. falciparum, P. vivax, P. ovale, or P. malariae (chloroquine-resistant) infections, a dose of 750 mg mefloquine followed by 500 mg 12 hours later is recommended. The pediatric dose of mefloquine is 15 mg/kg (less than 45 kg) followed by 10 mg/kg 8 to 12 hours later. Mefloquine is associated with sinus bradycardia, confusion, hallucinations, and psychosis and should be avoided in patients with a history of cardiovas cular problems or depression. IV quinidine gluconate followed by quinine plus doxycycline or clindamycin should be administered for severe illness as indicated above. The IV quinidine regimen requires close monitoring of the ECG (QT-segment) and other vital signs (hypotension and hypoglycemia). An alternative oral treatment for P. falciparum infections in adults, especially those with history of seizures, psychiatric disorders, or cardiovascular problems, is the combination of atovaquone 250 mg and proguanil 100 mg (Malarone) (two tablets twice daily for 3 days).12 The pediatric dose of Malarone is as follows: child less than 5 kg: not indicated; 9 to 10 kg: 3 pediatric tablets/day × 3 days; 11 to 20 kg: one adult tablet/day × 3 days; 21 to 30 kg: 2 adult tablets/day 3 days; 31 to 40 kg: 3 adult tablets/day 3 days; greater than 40 kg: 2 adults tablets twice daily × 3 days. ![]() Since falciparum malaria is associated with serious complications, including pulmonary edema, hypoglycemia, jaundice, renal failure, confusion, delirium, seizures, coma, and death; careful monitoring of fluid status and hemodynamic parameters is mandatory. Exchange transfusion that may be required in patients with P. falciparum malaria in whom parasitemia may be between 5% and 15% remains a questionable modality.57 Either peritoneal or hemodialysis may be indicated in renal failure.
Since falciparum malaria is associated with serious complications, including pulmonary edema, hypoglycemia, jaundice, renal failure, confusion, delirium, seizures, coma, and death; careful monitoring of fluid status and hemodynamic parameters is mandatory. Exchange transfusion that may be required in patients with P. falciparum malaria in whom parasitemia may be between 5% and 15% remains a questionable modality.57 Either peritoneal or hemodialysis may be indicated in renal failure.
Table 78–1 Chemoprophylaxis for Malaria
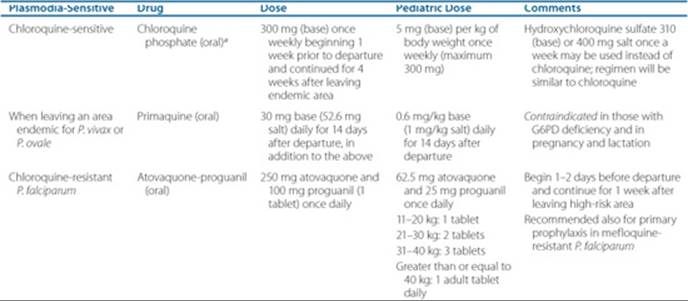
Patient Encounter 3, Part 2: Falciparum Malaria
TW presents with fever, nausea, headache, myalgias, chills, and body aches including back pain. When questioned about his travels, he indicates that he had not taken any antimalarial prophylaxis.
PMH: Healthy 27-year-old male
FH: Father died of stroke at age 87 years; mother, who is 82-year-old, has rheumatoid arthritis and lives with an unmarried daughter
SH: Systems analyst, works for local school district; occasionally drinks wine with meals
Meds: Ibuprofen 200 mg
ROS: In addition to the complaints noted above, he complains of severe nausea and fatigue
PE:
Gen: Patient is lucid but slightly agitated and febrile
VS: BP 105/70 mm Hg; P 120 bpm, RR 32 per minute, T 40.1°C (104.2°F)
Skin: Warm and dry to touch
HEENT: Slightly icteric sclerae and dry oral mucosa
ABD: Soft with diffuse tenderness with hepatomegaly and splenomegaly Rest of the systems were WNL
Labs: Sodium 131 mEq/L (131 mmol/L); hemoglobin 10.2 g/dL (102 g/L or 6.3 mmol/L); potassium 4.9 mEq/L (4.9 mmol/L); hematocrit 31% (0.31); chloride 96 mEq/L (96 mmol/L); WBC 14.8 × 103/mm3(14.8 × 109/L); BUN 28 mg/dL (10 mmol/L); total bilirubin 1.8 mg/dL (30.8 μmol/L); Scr 1.4 mg/dL (124 μmol/L); platelets 110 × 103/mm3 (110 × 109/L); glucose 77 mg/dL (4.27 mmol/L); aspartate aminotransferase 87 units/L (1.45 μkat/L); albumin 3.2 g/dL (32 g/L); alanine aminotransferase 94 units/L (1.57 μkat/L); blood smear (Giemsa stain): P. falciparum
In view of the above information, what is your assessment of this patient?
Identify your treatment goals and monitoring parameters.
OUTCOME EVALUATION
When advising potential travelers on prophylaxis for malaria, be aware of the incidence of chloroquine-resistant P. falciparum malaria and the countries where it is prevalent.60–74 In patients who have P. vivaxor P. ovale malaria (note that some patients can have P. falciparum and one of these species), following the treatment of the acute phase of malaria and screening for glucose-6-phosphate dehydrogenase deficiency, patients should receive a regimen of primaquine for 14 days to ensure eradication of the hypnozoite stage of P. vivax or P. ovale.63 For detailed recommendations for prevention of malaria go to www.cdc.gov/travel/.
Patient Encounter 3, Part 3: Malaria
Following treatment of falciparum malaria, TW has remained well for 2 months. However, 2 days ago, he started developing fever and chills, nausea, and abdominal pain. When seen in the emergency department he has a fever of 38.4°C (101.1°F) and complains of severe headache. Examinations of a thick and thin blood smear of the patient’s blood identified P. vivax infection. TW received a course of chloroquine and primaquine. In a follow-up 2 weeks later, a repeat blood smear was negative for parasites and the patient was asymptomatic.
Patient Care and Monitoring: Malaria
• Acute P. falciparum malaria resistant to chloroquine should be treated with IV quinidine via central venous catheter and fluid status and the electrocardiogram (ECG) should be monitored closely.
• The loading dose of quinidine should be omitted in those patients who have received quinine or mefloquine.
• Hypoglycemia that is associated with both P. falciparum and quinidine administration, should be checked every 4 to 6 hours and corrected with dextrose infusions (5–10%).
• Quinidine infusions should be slowed temporarily or stopped if the QT interval is greater than 0.6 second, the increase in the QRS complex is greater than 25%, or hypotension unresponsive to fluid challenge results.
• The suggested quinidine levels should be maintained at 3 to 7 mg/dL (9.2–21.6 μmol/L).
• Blood smears should be checked every 12 hours until parasitemia is less than 1%.
• Resolution of fever should take place between 36 and 48 hours after initiation of the IV quinidine therapy, and the blood should be clear of parasites in 5 days.
• When parenteral therapy is required for more than 48 hours or the patient’s renal function deteriorates, the dose of quinidine should be lowered by half.
Advice to Travelers
All travelers to endemic areas should be advised to remain in well-screened areas, to wear clothes that cover most of the body, and sleep in mosquito nets. Travelers should adhere to malaria chemoprophylaxis regimens and carry the insect repellant DEET (N, N-diethylmetatoluamide) or other insect sprays containing DEET for use in mosquito-infested areas.
AMERICAN TRYPANOSOMIASIS
ETIOLOGY
Two distinct forms of the genus Trypanosoma occur in humans. One is associated with African trypanosomiasis (sleeping sickness) and the other with American trypanosomiasis (Chagas’ disease). T. brucei gambiense and T. brucei rhodesiense are the causative organisms for the East African and West African trypanosomiasis, respectively. T. brucei rhodesiense causes the acute disease and is the more virulent of the two species. Both East and West African trypanosomiasis are transmitted by various species of tsetse fly belonging to the genus Glossina. Further discussion of this subject will focus on American trypanosomiasis.
T. cruzi is the agent that causes American trypanosomiasis. American trypanosomiasis is transmitted by a number of species of reduviid bugs (Triatoma infestans and Rhodrium prolixus) that live in wall cracks of houses in rural areas of North, Central, and South America.75–79 The reduviid bug is infected by sucking blood from animals (e.g., opossums, dogs, and cats) or humans infected with circulating trypomastigotes. American trypanosomiasis is endemic in all Latin American countries and can be transmitted congenitally, by blood transfusion, and by organ transplantation.
Clinical Presentation and Diagnosis of Trypanosomiasis
Acute
• Unilateral orbital edema (Romana’s sign)
• Granuloma (chagoma)
• Fever, hepatosplenomegaly, and lymphadenopathy
Chronic ![]()
• Cardiac: cardiomyopathy and heart failure
• ECG: first-degree heart block, right bundle-branch block, and arrhythmias
• GI: enlargement of the esophagus and colon (“mega” syndrome)
• CNS: meningoencephalitis, strokes, seizures, and focal paralysis
Diagnosis
Positive history of exposure and use of serology: indirect hemagglutination test, ELISA (Chagas EIA, Abbott Labs, Abbott Park, IL), and complement fixation (CF) test. (Note: CF may produce false-positive reactions in those exposed to leishmaniasis, syphilis, and malaria. PCR may be more definitive for diagnosis.)
Patient Care and Monitoring
• It is essential to identify T. cruzi–infected patients by serology and to monitor the cardiovascular status of these patients by ECG periodically.
• Some patients will benefit from implantation of pacemakers.
• All transplant candidates from areas endemic for Chagas’ disease need to be screened for T. cruzi. Immunosuppression in these patients can lead to overwhelming infections.
CLINICAL PRESENTATION AND DIAGNOSIS
Pharmacologic Therapy
The drugs used for T. cruzi include nifurtimox (Lampit) and benznidazole (Rochagan). Oral nifurtimox is available from the CDC, while benznidazole is only available in Brazil.12,80–83 The adult dose of nifurtimox is 8 to 10 mg/kg/day in divided doses for 120 days. Since children seem to tolerate the dose better than adults, the pediatric dose of nifurtimox in children 1- to 10-year-old is 15 to 20 mg/kg/day, and the dose for children 11- to 16-year-old is 12.5 to 15 mg/kg/day in divided doses. Symptomatic treatment for heart failure associated with Chagas’ disease should be initiated. The GI complications may require surgical revisions and reconstruction.
OUTCOME EVALUATION
Treatment of the acute phase of the disease (i.e., fever, malaise, edema of the face, and hepatosplenomegaly) is nifurtimox. The congestive heart failure associated with cardiomyopathy of Chagas’ disease is treated the same way as cardiomyopathy from other causes.76,80
ECTOPARASITES
A parasite that lives outside the body of the host is called an ectoparasite. Approximately 6 to 12 million subjects become infested with pediculosis (lice infestation) yearly in the United States. Pediculosis is usually associated with poor hygiene, and infections are passed from person to person through social and sexual contact.
LICE
The two species that belong to this group include Pediculus humanus capitis (head louse) and P. humanus corporis (body louse).84–88 The eggs (or nits) remain firmly attached to the hair, and in about 10 days the lice hatch to form nymphs, which mature in 2 weeks. The lice become attached to the base of the hair follicle and feed on the blood of the host.10 Pubic or crab lice is found on the hairs around the genitals but may occur in other parts of the body (e.g., eyelashes or axillae). Hypersensitivity to the secretions from lice can produce macular swellings and lead to secondary bacterial infections.
Treatment
![]() The agent of choice for all three infections (body, head, and crab lice) is 1% permethrin (Nix). Permethrin has both pediculicidal and ovicidal activity against P. humanus capitis. The cure rate is reported to be between 90% and 97%. A cream rinse of permethrin 1% (Nix-Crème Rinse) is also available. Individuals with a history of hypersensitivity to ragweed or chrysanthemum may react to permethrin and should avoid this preparation. An alternative agent is oral ivermectin 100 mcg/kg for 3 days (days 1, 2, and 10). Permethrin can cause itching, burning, stinging, and tingling with application. Permethrin 1% should be applied to the dry scalp after shampooing and be left on the scalp for 10 minutes. The application may need to be repeated. Because of the reports of resistance to permethrin, an alternative agent is 0.5% malathion (Ovide), which has to be left on the scalp for 90 minutes and has also been found to be effective. For the relief of pruritus, calamine lotion with 0.1% menthol or an equivalent agent may be used. All individuals, including immediate family members and sexual partners of the primary host, should be treated. All bedding and clothes should be sterilized as previously indicated for enterobiasis.
The agent of choice for all three infections (body, head, and crab lice) is 1% permethrin (Nix). Permethrin has both pediculicidal and ovicidal activity against P. humanus capitis. The cure rate is reported to be between 90% and 97%. A cream rinse of permethrin 1% (Nix-Crème Rinse) is also available. Individuals with a history of hypersensitivity to ragweed or chrysanthemum may react to permethrin and should avoid this preparation. An alternative agent is oral ivermectin 100 mcg/kg for 3 days (days 1, 2, and 10). Permethrin can cause itching, burning, stinging, and tingling with application. Permethrin 1% should be applied to the dry scalp after shampooing and be left on the scalp for 10 minutes. The application may need to be repeated. Because of the reports of resistance to permethrin, an alternative agent is 0.5% malathion (Ovide), which has to be left on the scalp for 90 minutes and has also been found to be effective. For the relief of pruritus, calamine lotion with 0.1% menthol or an equivalent agent may be used. All individuals, including immediate family members and sexual partners of the primary host, should be treated. All bedding and clothes should be sterilized as previously indicated for enterobiasis.
SCABIES
![]() Scabies is caused by the itch mite Sarcoptes scabiei hominis, which affects both humans and animals. Infection usually affects the interdigital and popliteal folds, axillary folds, the umbilicus, and the scrotum. The infection causes severe itching and excoriations in the interdigital web spaces, buttocks, groin, and scalp.89,90 Diagnosis is made by identifying the mite from skin scrapings on a wet mount.
Scabies is caused by the itch mite Sarcoptes scabiei hominis, which affects both humans and animals. Infection usually affects the interdigital and popliteal folds, axillary folds, the umbilicus, and the scrotum. The infection causes severe itching and excoriations in the interdigital web spaces, buttocks, groin, and scalp.89,90 Diagnosis is made by identifying the mite from skin scrapings on a wet mount.
Treatment
![]() The agent of choice for scabies is permerthrin 5% (Elimite) cream. Alternative agents in subjects who cannot use permethrin are crotamiton 10% (Eurax) and oral ivermectin (Stromectal) 200 mcg/kg as a single dose. To initiate the treatment with permethrin, the skin should be scrubbed in a warm soapy bath to remove the scabs. The permethrin lotion should then be applied to the whole body, avoiding the face, mucous membranes, and eyes, and left on for 8 to 14 hours. A single application eradicates 97% of scabies. All close contacts should be treated appropriately. The pruritus associated with scabies may persist for 2 to 4 weeks because of the remnants of mite parts in the skin.
The agent of choice for scabies is permerthrin 5% (Elimite) cream. Alternative agents in subjects who cannot use permethrin are crotamiton 10% (Eurax) and oral ivermectin (Stromectal) 200 mcg/kg as a single dose. To initiate the treatment with permethrin, the skin should be scrubbed in a warm soapy bath to remove the scabs. The permethrin lotion should then be applied to the whole body, avoiding the face, mucous membranes, and eyes, and left on for 8 to 14 hours. A single application eradicates 97% of scabies. All close contacts should be treated appropriately. The pruritus associated with scabies may persist for 2 to 4 weeks because of the remnants of mite parts in the skin.

Self-assessment questions and answers are available at http://www.mhpharmacotherapy.com/pp.html.
OUTCOME EVALUATION
Infections due to arthropods can be controlled by preventing their access to the host. Improving living conditions and avoiding sharing common personal items like hats and hair brushes may minimize these infections due to arthropods. Permethrin (1–5%) is an effective agent for all these infections.
Abbreviations Introduced in This Chapter

REFERENCES
1. Garg PK, Perry S, Dorn M, et al. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg 2005;73:386–391.
2. White AC, Atmar RL. Infections in Hispanic immigrants. Clin Infect Dis 2002;34:1627–1632.
3. Bledsoe GH. Malaria primer for clinicians in the United States. South Med J 2005;98:1197–1204.
4. Chen LH, Wilson ME, Schlagenhauf P. Controversies and misconceptions in malaria chemoprophylaxis for travelers. JAMA 2007;297:2251–2263.
5. Malaria Surveillance—United States, 2007. MMWR Surveill Summ 2009;58(SS-02):1–16.
6. Vicas AE, Albrecht H, Lennox JL, del Rio C. Imported malaria at an inner-city hospital in the United States. Am J Med 2005;329:6–12.
7. Franco-Paredes C, Santos-Preciado JI. Problem pathogens: Prevention of malaria in travelers. Lancet Infect Dis 2006;6:139–149.
8. Nuesch R, Zimmerli L, Stockli R, et al. Imported Strongyloidosis: A longitudinal analysis of 31 cases. J Travel Med 2005;29:80–84.
9. John DT, Petri WA Jr. Markell and Voge’s Medical Parasitology, 9th ed. Philadelphia: Saunders, 2006.
10. Escobedo AA, Cimerman S. Giardiasis: A pharmacotherapy review. Expert Opin Pharmacother 2007;8(12):1885–1902.
11. Hill DR. Giardia lamblia. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases, 7th ed. New York: Elsevier Churchill-Livingstone, 2009:3527–3534.
12. Drugs for parasitic infections. In: Handbook of Antimicrobial Therapy, 18th ed. New Rochelle, NY: Medical Letter, 2008:225–280.
13. Lebwohl B, Deckelbaum RJ, Green PHR. Giardiasis. Gastrointest Endosc 2003;57:906–913.
14. Huang DB, White AC. An updated review on Cryptosporium and Giardia. Gastroenterol Clin North Am 2006;35:291–314.
15. Sorell L, Garrote JA, Galvan JA, et al. Celiac disease diagnosis in patients with giardiasis: High value of antitransglutaminase antibodies. Am J Gastroenterol 2004;99:1330–1332.
16. Nitazoxanide (alina): A new antiprotozoal agent. Med Lett Drugs Ther 2003;45:29–31.
17. Hung C-C, Deng H-Y, Hsiao W-H, et al. Invasive amebiasis as an emerging parasite disease in patients with human immunodeficiency virus type 1 infection in Taiwan. JAMA 2005;165:409–415.
18. Farthing MJG. Intestinal protozoa. Entamoeba histolytica. In: Manson’s Tropical Diseases, 22nd ed. London: WB Saunders, 2009: 1375–1386.
19. Haque R, Huston CD, Hughes M, et al. Amebiasis. N Engl J Med 2003;348:1565–1573.
20. Petri Jr WA, Haque R. Entamoeba histolytica (amebiasis). In: Mandell GL, Bennett JA, Dolin R, eds. Principles and Practice of Infectious Diseases, 7th ed. New York: Elsevier Churchill-Livingstone, 2009:3411–3425.
21. Salles JM, Morales LA, Salles MC. Hepatic Amebiasis. Braz J Infect Dis 2003;7:96–110.
22. Bercu TE, Petri Jr WA, Behm BW. Amebic colitis: New insights into pathogenesis and treatment. Curr Gastroenterol Rep 2007;9:429–433.
23. Ozdogan M, Baykal A, Aran O. Amebic perforation of the colon: Rare and frequently fatal complication. World J Surg 2004;28:926–929.
24. Maguire JH. Intestinal nematodes (roundworms). In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases, 7th ed. New York: Elsevier Churchill-Livingstone, 2009:3577–3586.
25. Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infection: Ascariasis, trichuriasis, and hookworm. Lancet 2006;367: 1521–1532.
26. Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med 2004;357:799–807.
27. Gabriella AF, Ramsan M, Naumann C, et al. Soil-transmitted helminthes and haemoglobin status among Afghan children in World Food Programme Assisted Schools. J Helminthol 2005;79:381–384.
28. Larocque R, Casapia M, Gotuzzo E, Gyorkos TW. Relationship between intensity of soil-transmitted helminth infections and anemia in pregnancy. Am J Trop Med Hyg 2005;73:783–789.
29. Sahoo PK, Satapathy AK, Michael E, Ravindran B. Concomitant parasitism: Bancroftian filariasis and intestinal helminthes and response to albendazole. Am J Trop Med Hyg 2005;73:877–880.
30. Malik AH, Saima BD, Wani MY. Management of hepatobiliary and pancreatic ascariasis in children of endemic area. Pediatr Surg Int 2006;22:164–168.
31. Huratado RM, Sahani DV, Kradin RL. Case records of the Massachusetts General Hospital. Case 9-2006. A 35-year-old woman with recurrent-upper-quadrant pain. N Engl J Med 2006;354:1295–1303.
32. Petro M, Iavu K, Minocha A. Unusual endoscopic and microscopic view of Enterobius vermicularis: A case report with review of the literature. South Med J 2005;98:927–929.
33. Keiser PB, Nutman TB. Strongyloides stercoralis in the immuno-compromised population. Clin Microbiol Rev 2004;17:208–217.
34. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: Risk factors, diagnosis and management. Can Med Assoc J 2004;171:479–484.
35. Schaeffer MW, Buell JF, Gupta M, et al. Strongyloides hyperinfection syndrome after heart transplantation: Case report and review of literature. J Heart Lung Transplant 2004;23:905–911.
36. Concho R, Harrington W, Rogers AI. Intestinal Strongyloidiasis: Recognition, management, and determinants of outcome. Clin Gastroenterol 2005;39:203–211.
37. Newberry AM, Williams DN, Stauffer WM, et al. Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis. Chest 2005;128:3681–3684.
38. Satoh M, Kokaze A. Treatment strategies in controlling strongyloidiasis. Expert Opin Pharmacother 2004;5:2293–2301.
39. Muennig P, Pallin D, Challah C, Khan K. The cost-effectiveness of ivermectin vs albendazole in the presumptive treatment of strongyloidiasis in immigrants to the United States. Epidemiol Infect 2004;132:1055–1063.
40. King CH. Cestodes (tapeworms). In: Mandell GL, Dolin R, Bennett JE, eds. Principles and Practice of Infectious Diseases, 7th ed. New York: Elsevier Churchill-Livingstone, 2009:3607–3616.
41. Garcia HH, Gonzalez AE, Evans CAW, Gilman RH. Taenia solium cysticercosis. Lancet 2003;361:547–556.
42. Del La Garza Y, Graviss EA, Daver NG, et al. Epidemiology of neurocysticercosis in Houston, Texas. Am J Trop Med Hyg 2005;73:766–770.
43. Townes JM, Hoffmann CJ, Kohn MA. Neurocysticercosis in Oregon, 1995-2000. Emerging Infect Dis 2004;10:508–510.
44. Dua T, Aneja S. Neurocysticercosis: Management issues. Indian Pediatr 2006;43:227–235.
45. Garcia HH, Del Brutto OH, Nash TE, et al. New concepts in the diagnosis and management of neurocysticercosis (Taenia solium). Am J Trop Med Hyg 2005;72:3–9.
46. Gongora-Rivera F, Soto-Hernandez JL, Esquivel DG, et al. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology 2006;66:436–438.
47. Fairhurst RM, Wellems TE. Plasmodium species (malaria). In: Mandell GL, Dolin R, Bennett JE, eds. Principles and Practice of Infectious Diseases, 7th ed. New York: Elsevier Churchill-Livingstone, 2009:3437–3462.
48. White NJ, Breman JG. Malaria and babesiosis: Diseases caused by red blood cell parasites. In: Harrison’s Principles of Internal Medicine, 18th ed. New York: McGraw-Hill, 2008:1280–1294.
49. Newman RD, Parise ME, Barber AM, Steketee RW. Malaria-related deaths among travelers, 1963-2001. Ann Intern Med 2004;141:547–555.
50. Kitchen AD, Chiodini PL. Malaria and blood transfusion. Vox Sang 2006;90:77–84.
51. White NJ. Malaria. In: Cook GC and Zumla A, eds. Manson’s Tropical Diseases, 21st ed. London: WB Saunders, 2003:1205–1295.
52. Idro R, Carter JA, Fegan G, et al. Risk factors for persisting neurological and cognitive impairment following cerebral malaria. Arch Dis Child 2006;91:142–148.
53. Idro R, Jenkins NE, Newton CRJC. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol 2005;4:827–840.
54. Pasvol G. Management of severe malaria: Interventions and controversies. Infect Dis Clin North Am 2005;19:211–240.
55. Eiam-Ong S. Malarial nephropathy. Semin Nephrol 2003;23:21–33.
56. Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical Review: Severe Malaria. Crit Care 2003;7:315–323.
57. Riddle MS, Jackson JL, Sanders JW, Blazes DL. Exchange transfusion as an adjunct therapy in severe Plasmodium falciparum malaria: A meta-analysis. Clin Infect Dis 2002;34:1192–1198.
58. Taylor WRJ, White NJ. Malaria and the lung. Clin Chest Med 2002;23:457–468.
59. Singh N, Saxena A. Usefulness of a rapid on-site plasmodium falciparum diagnosis (PARACHECK® PF) in forest migrants and among the indigenous population at the site of their occupational activities in central India. Am J Trop Med Hyg 2005;72:26–29.
60. Magill AJ. The prevention of malaria. Prim Care 2002;29:815–842.
61. Chen LH, Wilson ME, Schlagenhauf P. Prevention of malaria in long-term travelers. JAMA 2006;296:2234–2244.
62. Shanks GD, Edstein MD. Modern malaria chemoprophylaxis. Drugs 2005;65:2091–2110.
63. Taylor WRJ, White NJ. Antimalarial drug toxicity. Drug Saf 2004;27:25–61.
64. Tako EA, Zhou A, Lohoue J, et al. Risk factors for placental malaria and its effect on pregnancy outcome in Yaounde, Cameroon. Am J Trop Med Hyg 2005;72:236–242.
65. Sharma S, Pathak S. Malaria vaccine: A current perspective. J Vector Borne Dis 2008;45:1–20.
66. Ballou WR, Arevalo-Herrera M, Carucci D, et al. Update on the clinical development of candidate malaria vaccines. Am J Trop Med Hyg 2004;71:239–247.
67. Alonso P, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS, S/ASO2A vaccine against Plasmodium infection and disease in young African children: Randomized controlled trial. Lancet 2004;364:1411–1420.
68. Bacaner N, Stauffer B, Boulware DR, Walker PF, Keystone JS. Travel medicine considerations for North American immigrants visiting friends and relatives. JAMA 2004;291:2856–2864.
69. Rathore D, McCutchan TF, Sullivan M, Kumar S. Antimalarial drugs: Current status and new developments. Expert Opin Investig Drugs 2006;14:871–883.
70. Chen LH, Keystone JS. New strategies for the prevention of malaria in travelers. Infect Dis Clin North Am 2005;19:185–210.
71. Rosenthal PJ. Artesunate for the treatment of severe Falciparum malaria. N Engl J Med 2008;358:1829–1836.
72. Price RN, Uhlemann A-C, van Vugt M, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multi-resistant Plasmodium falciparum malaria. Clin Infect Dis 2006;42:1570–1577.
73. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med 2002;347:13–18.
74. Rosenthal PJ. Antiprotozoal drugs. In: Katzung BG, ed. Basic and Clinical Pharmacology, 11th ed. New York: Lange Medical Books/McGraw-Hill, 2009:899–921.
75. Barrett MP, Burchmore RJS, Stich A, et al. The trypanosomiasis. Lancet 2003;362:1469–1480.
76. Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. Pathogenesis of chronic Chagas heart disease. Circulation 2007;115:1109–1123.
77. Kirchhoff LV. Trypanosoma species (American trypanosomiasis, Chagas’ disease): Biology of trypanosomes. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases, 7th ed. New York: Elsevier Churchill-Livingstone, 2009:3481–3488.
78. Kirchhoff LV. American trypanosomiasis (Chagas disease). In: Guerrant RL, Walker H, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens, and Practice, 2nd ed. 2006:1082–1094.
79. Carod-Artal FJ, Vargas AP, Melo M, Horan TA. American trypanosomiasis (Chagas’ disease): An unrecognized cause of stroke. J Neurol Neurosurg Psychiatry 2003;74:516–518.
80. Schijman AG, Vigliano CA, Viotti RJ, et al. Trypanosoma cruzi DNA in cardiac lesions of Argentine patients with end-stage chronic Chagas heart disease. Am J Trop Med Hyg 2004;70:210–220.
81. Chagas disease after organ transplantation – Los Angeles, California, 2006. MMWR Morb Mortal Wkly Rep 2006;55:798–800.
82. Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment. A nonrandomized trial. Ann Intern Med 2006;144:724–734.
83. Rosenthal PJ. Clinical pharmacology of the anthelmintic drugs. In: Katzung BG, ed. Basic and Clinical Pharmacology, 11th ed. New York: Lange Medical Books/McGraw-Hill, 2009:923–934.
84. Diaz SH. Lice (pediculosis). In: Mandell GL, Bennett JR, Dolin R, eds. Principles and Practice of Infectious Diseases, 7th ed. New York: Elsevier Churchill-Livingstone, 2009:3629–3632.
85. Roberts RJ. Head lice. N Engl J Med 2002;346:1645–1650.
86. Yoon KS, Gao J-R, Taplin D, et al. Permethrin-resistant human head lice, pediculus capitis, and their treatment. Arch Dermatol 2003; 139:994–1000.
87. Burkhart CG. Relationship of treatment-resistant head lice to the safety and efficacy of pediculicides. Mayo Clin Proc 2004;79:661–666.
88. Jones KN, English III JC. Review of common therapeutic options in the United States for the treatment of pediculosis capitis. Clin Infect Dis 2003;36:1355–1361.
89. Johnson G, Sladden M. Scabies: Diagnosis and treatment. Br Med J 2005;331:619–622.
90. Chosidow O. Scabies. N Engl J Med 2006;354:1718–1727.