Christopher B. Colwell and Ernest E. Moore
Shock is the ultimate consequence of inadequate tissue perfusion, which may be manifested clinically by hemodynamic disturbances or organ dysfunction. At the cellular level, shock initially results from the insufficient delivery of required metabolic substrates, principally oxygen, to sustain aerobic metabolism. In the setting of trauma, shock is most often related to loss of circulating blood volume caused by hemorrhage, although inadequate oxygenation, cardiac dysfunction, neurologic dysfunction, or mechanical vascular obstruction may be either primary or contributing factors.
There are four general pathophysiologic types of shock: (a) hemorrhagic or hypovolemic, (b) cardiogenic, (c) neurogenic or vasogenic, and (d) septic. Hemorrhagic shock involves the loss of circulating intravascular volume caused by blood loss internally, externally, or both. Cardiogenic shock indicates a process that prevents the normal pumping of the heart and can be caused by pericardial tamponade that prevents normal ventricular filling, tension pneumothorax with vena caval compression and reduction in venous return to the heart, or direct cardiac damage with loss of contractile force (myocardial contusion).
Neurogenic or vasogenic shock may result from spinal cord injury with loss of peripheral vascular resistance. Major spinal cord injury results in acute vasodilatation but generally does not cause impaired tissue perfusion unless other injuries are present. Spinal cord injuries result in a loss of sympathetic tone and are, therefore, accompanied by bradycardia (spinal shock). Septic shock refers to a hyperdynamic response (elevated cardiac output and low systemic vascular resistance), followed by decreased cardiac output and increased systemic vascular resistance, eventually resulting in organ function deterioration associated with the inflammatory mediators accompanying infection.
If the acute stress of the traumatic shock state is sufficiently severe or prolonged, organ dysfunction may also develop, including acute kidney injury (AKI), adult respiratory distress syndrome (ARDS), and multiple organ failure (MOF). These entities can develop within a few hours to several weeks after the acute injury.
Shock is a common and potentially treatable cause of death in injured patients. Traumatic shock usually results from one or more of the following mechanisms: (a) acute blood loss from solid organ injury, rib fractures, major vascular injury, pelvic fracture, or multiple long-bone fractures; (b) cardiac dysfunction from blunt cardiac injury, tension pneumothorax, or pericardial tamponade; (c) hypoxemia caused by airway compromise or pulmonary contusion; or (d) loss of vasomotor integrity (distributive) caused by neurologic injury and late overwhelming infection.
The pathophysiology of traumatic shock is largely related to an imbalance in oxygen supply and demand. In the early phase of acute trauma, this imbalance is often caused by hypoperfusion, although low arterial blood oxygen saturation may be a significant contributing factor. Aggressive resuscitation with fluids having low or no oxygen-carrying capacity in the setting of severe and ongoing hemorrhage may lead to a critically low hemoglobin concentration that further contributes to tissue oxygen debt.
Acute blood loss elicits compensatory hemodynamic changes that serve to maintain vital organ perfusion. These changes include increased heart rate and vasoconstriction primarily in the peripheral circulation and splanchnic vascular beds. The term compensated shock refers to varying degrees of tachycardia and peripheral vasoconstriction that maintain adequate critical organ perfusion. Arterial blood pressure in compensated shock states may be moderately low or within the normal range. The term uncompensated shock indicates inadequate vital organ perfusion.
Vasoconstriction in response to acute blood loss is a protective response that preserves perfusion to the vital organs at the expense of peripheral and abdominal organ perfusion. In the hypoperfused tissues, oxygen demand exceeds oxygen delivery, and the numerous cellular processes that depend on oxidative metabolism and subsequent production of adenosine triphosphate (ATP) begin to fail. Acidosis results when aerobic metabolism slows, as protons cannot be used to synthesize ATP from adenosine diphosphate (ADP); lactate cannot be converted to pyruvate and, therefore, lactate increases. If the pathologic processes are not corrected, cellular dysfunction and eventually organ failure result.
CLINICAL PRESENTATION
Initial findings in a patient with traumatic shock may include tachycardia, hypotension, signs of poor peripheral perfusion, and alteration in mental status. In response to a loss of circulating volume, the heart rate will increase to sustain normal cardiac output. Continued blood loss will eventually result in a decrease in blood pressure. Peripheral vasoconstriction and central venoconstriction shunt blood centrally and result in a narrowed pulse pressure. Clinically, decreases in peripheral perfusion manifest as cool, pale, clammy extremities with prolongation of capillary refill (Table 20.1).
TABLE 20.1
Classes of Hemorrhagic Shock
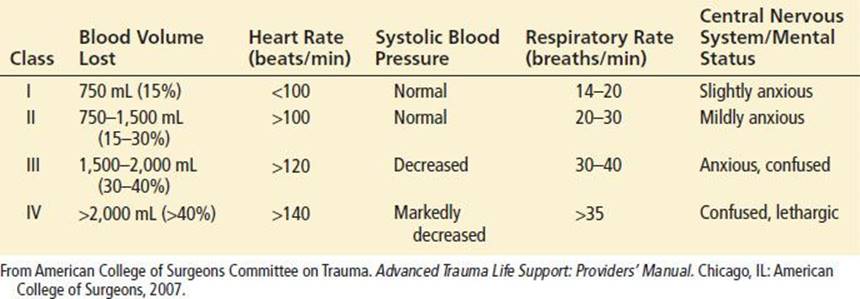
The skin may become mottled in appearance, but cyanosis is not a common finding. The narrowed pulse pressure can make the pulse quality weak or thready. Alterations in mental status caused by hypoperfusion may be subtle initially and can be difficult to distinguish from associated head injury or intoxication. Therefore, altered mental status on presentation or a subsequent decline in mental status, especially in patients without head trauma, should raise concern of systemic hypoperfusion and impending circulatory collapse.
Shock may be present even in the setting of “normal” vital signs. Young, healthy patients with substantial blood loss may maintain a blood pressure within the normal range by compensatory vasoconstriction and increased heart rate. Heart rate can occasionally be in the upper normal range. In such patients, signs of peripheral hypoperfusion and subtle changes in mental status may be the only warning signs preceding rapid hemodynamic decompensation. Elderly patients may not develop a tachycardic response to blood loss because of pre-existing medical conditions or medications. In fact, a bradycardic response may occur with rapid blood loss that is believed to be vagally mediated. Conversely, hypotension and tachycardia may be exacerbated in a pregnant trauma patient for a given degree of blood loss caused by compression of the inferior vena cava by the gravid uterus, resulting in decreased venous return.
Decline in urine output caused by renal hypoperfusion and renal fluid reabsorption is an important manifestation of shock physiology. Monitoring urine output may be useful in the emergency department (ED); later, it is a critical parameter in the surgical intensive care unit. Therefore, a urinary catheter should be placed in patients who exhibit evidence of shock, and the bladder should be drained to begin urine-output monitoring and to check for hematuria.
DIFFERENTIAL DIAGNOSIS
The most common cause of postinjury shock is hemorrhage-induced hypovolemia. However, several pathologic entities must be considered as either potential contributors or primary causes of shock. These entities are listed in Table 20.2.
TABLE 20.2
Causes of Shock

Pericardial tamponade is classically described as exhibiting the Beck triad of hypotension, distended neck veins, and muffled heart sounds. A pulsus paradoxus of >10 mm Hg may also be seen. However, not all of these markers may be present or detected. In a hypovolemic patient, the neck veins may be flat. Muffled heart sounds may be difficult to distinguish in a busy trauma resuscitation room, and detection of a pulsus paradoxus is not reliable. Pericardial tamponade should be considered whenever there is penetrating chest trauma, when there are rib fractures near the heart, or when there has been significant blunt trauma to the chest. The immediate availability of ultrasonography or echocardiography offers the best potential for rapid and accurate diagnosis.
A large pneumothorax or hemothorax can usually be detected by diminished breath sounds, although auscultation can be unreliable. Ultrasound (US) is useful for confirmation with a chest radiograph warranted if the US is equivocal. A tension pneumothorax may also result in deviation of the trachea away from the affected side, cardiac displacement, and hypotension related to inferior vena caval compression that limits venous return to the heart. However, tracheal deviation and cardiac displacement are late findings and may be difficult to detect by physical examination. Hypoxemia may be an earlier sign of a tension pneumothorax than hypotension. If a patient is hypotensive and has clinical evidence of a pneumothorax, placement of a chest tube before diagnostic imaging is appropriate.
Blunt cardiac injury (formally referred to as myocardial contusion) resulting in significant contractile dysfunction is a difficult diagnosis to make in the ED unless echocardiography is immediately available. Blunt cardiac injury should be suspected when blunt trauma involves the sternum and anterior left chest, particularly if ventricular ectopy, dysrhythmias, or electrocardiographic demonstration of ST-segment elevations (especially in the anterior precordial leads) are present. In addition, blunt trauma can result in valvular disruption and coronary artery dissection. Evidence of cardiogenic shock (hypotension, tachycardia, elevated central venous pressure) should alert the physician to the possible presence of a blunt cardiac injury or pericardial tamponade.
Spinal cord trauma with neurogenic shock may present with hypotension caused by loss of peripheral vascular resistance. In addition, because of the loss of sympathetic tone, these patients will not have the usual tachycardic response to hypotension but often demonstrate bradycardia instead. In this setting, the presence of neurologic deficits and lack of signs of peripheral vasoconstriction should arouse suspicion for this entity. Such patients generally have warm extremities and good urine output. Volume status must be carefully monitored, because excess fluid administration in patients with spinal shock may be detrimental.
Signs of shock in a trauma patient may not be a direct consequence of the injury. For example, a myocardial infarction may be the cause of a motor vehicle collision, or the physiologic stress associated with trauma may precipitate myocardial ischemia in a patient with underlying coronary artery disease. History, physical examination, or electrocardiographic findings consistent with myocardial infarction should arouse suspicion of combined medical and surgical processes. Another rare medical cause of hypotension in a trauma patient is anaphylaxis that may have preceded the trauma. Pharmacologic agents such as β-adrenergic blockers and recreational agents, such as ethanol and cocaine, may also significantly affect the clinical picture in the setting of acute trauma.
ED EVALUATION
The initial step in managing shock in the injured patient is prompt recognition. Effective management of the acutely injured patient exhibiting signs of shock requires that initial assessment and treatment begin simultaneously (1) with an emphasis on the airway, breathing, and circulation (ABCs). Assessment of airway patency, adequacy of ventilation (respiratory excursion and lung auscultation), hemodynamic status (pulse rate, central and peripheral pulse quality, blood pressure), and evidence of controllable hemorrhage should be immediately linked with interventions to (a) secure the airway while protecting the cervical spine, (b) enhance oxygenation, (c) provide ventilatory assistance, (d) limit further hemorrhage, (e) gain intravenous access, (f) initiate volume replacement, (g) obtain blood for laboratory and blood bank testing, and (h) perform US screening of the chest and abdomen (Fig. 20.1).
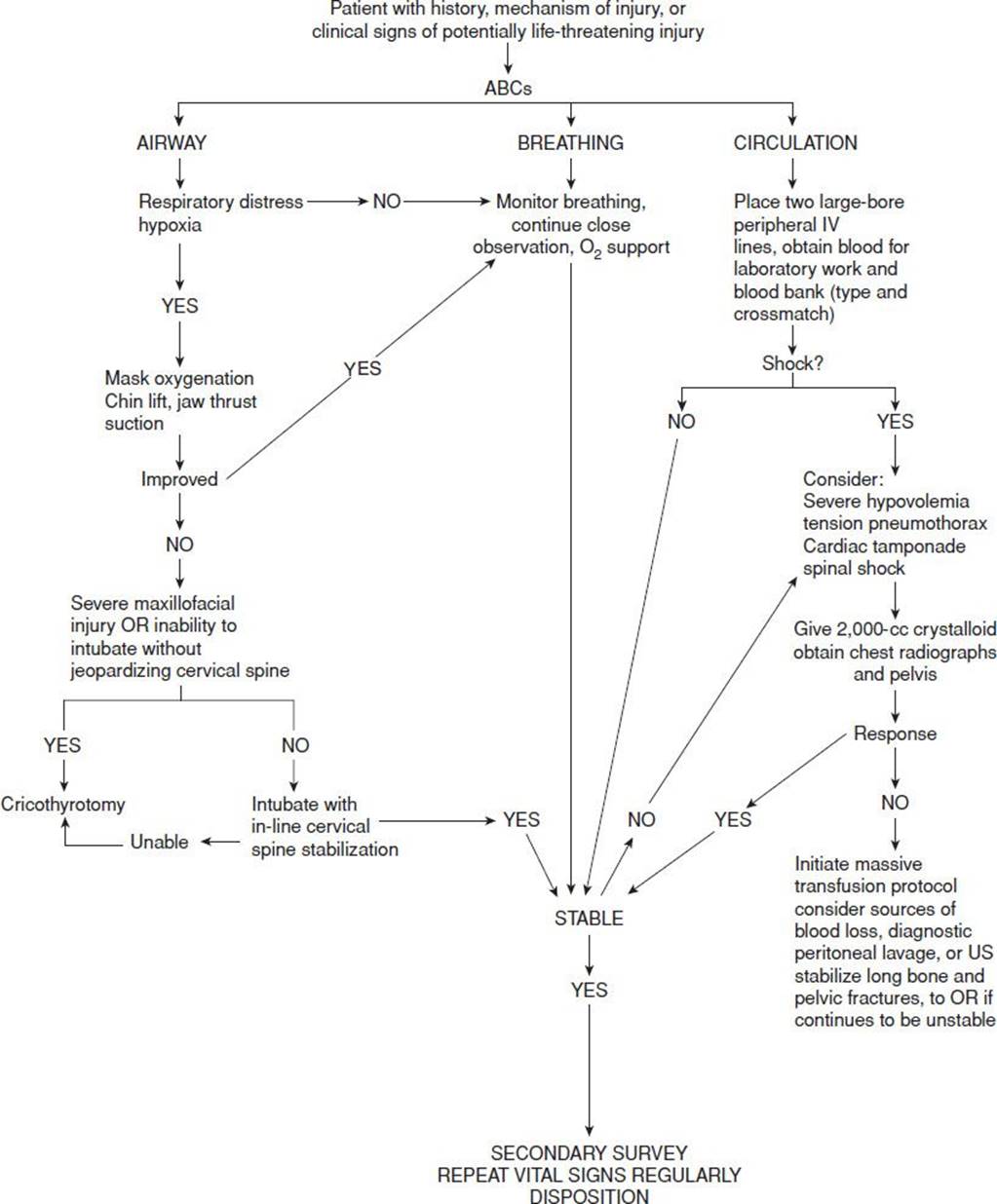
FIGURE 20.1 Treatment algorithm for traumatic shock.
After the primary assessment and initiation of treatment, a thorough secondary assessment should be conducted to identify potential injuries to the head, neck, chest, abdomen, pelvis, back, extremities, neurologic, and vascular system. Hypothermia can depress hemodynamics and aggravate coagulopathy and should be aggressively treated. Acute trauma is a highly dynamic disease process that requires frequent and careful reassessment of ventilation, hemodynamic status, and physical examination to optimally adjust therapeutic interventions and identify evolving pathologic processes.
Initial laboratory studies in patients with major trauma should include (a) complete blood count (CBC); (b) arterial blood gases (base deficit); (c) electrolytes; (d) coagulation studies; (e) type and cross match for four units of packed red blood cells (PRBCs); and (f ) toxicologic studies (Table 20.3). Urine should be obtained and checked for blood. Base deficit is a reliable early index of the magnitude of shock (2). Serum lactate has been advocated by some as an early predictor of morbidity and mortality, particularly in burn patients (3), but it appears to lag behind base deficit in the first 12 hours after injury (4).
TABLE 20.3
Initial Laboratory and Radiographic Studies in Major Trauma
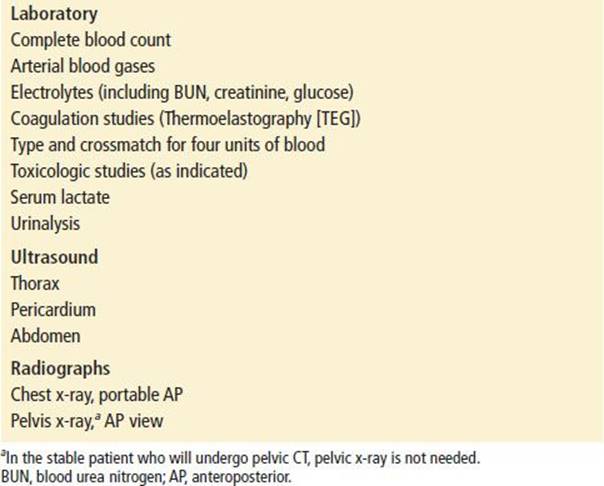
Initial radiographs have previously included the lateral view cervical spine, chest, and pelvis. With a number of studies suggesting that computed tomography (CT) scanning of the cervical spine is superior to plain films for detecting fractures (5), most centers are forgoing plain films of the cervical spine and going straight to CT. X-rays of the pelvis do not generally play a significant role in most trauma patients. Many of these patients will be getting a CT scan of the abdomen and pelvis and do not need a plain film unless they are unstable and may need to go directly to the operating room. In the unstable patient, a pelvic x-ray can determine the need for pelvic stabilization (by a bed sheet tied around the pelvis), as the patient is resuscitated initially or transferred to the operating room.
The initial chest radiograph will identify pneumothorax, hemothorax, pulmonary contusion, or a widened mediastinum, and the initial pelvic radiograph will identify major pelvic fractures as a potential cause of hypotension. Any patient with suspected spinal fractures or spinal shock should undergo radiographic evaluation once stabilized. The thoracic and lumbar spine can be evaluated by CT scanning of the chest and abdomen, if these studies are indicated. Otherwise CT scans of the spine are appropriate in most situations.
US is part of the initial evaluation of the trauma patient. During the initial resuscitation, the focused assessment with sonography for trauma (FAST) examination should be performed to obtain views of the heart and abdomen to assess for pericardial effusion and intraperitoneal bleeding. Serial US examinations can be performed to increase the sensitivity for detecting intraperitoneal bleeding. In addition, US can be used for prompt detection of a hemothorax or pneumothorax. An alternative means of detecting intraperitoneal bleeding is diagnostic peritoneal lavage (DPL). The unstable trauma patient should undergo DPL when US is not available or if the US results are equivocal.
Monitoring of heart rate, respiratory rate, blood pressure, temperature, and pulse oximetry are important. Early placement of central venous pressure lines should be considered in patients who do not stabilize hemodynamically or are suspected of having cardiogenic or neurogenic shock components. Placement of a nasogastric or orogastric tube for decompression reduces the chances of aspiration and may improve ventilation if the stomach is distended with air.
A urinary drainage catheter should be placed after a search for potential urethral injury has been performed.
Urethral injury should be suspected in patients with pelvic fractures in the area of the symphysis pubis, anterior lacerations on rectal or vaginal examination, or abnormal position of the prostate gland. As noted, urine output becomes an important indicator of the adequacy of organ perfusion.
Quantitative end-tidal carbon dioxide (ETCO2) monitoring is useful in intubated or sedated patients. ETCO2 can help avoid hypoventilation, prevent excessive therapeutic hyperventilation, and detect decreases in cardiac output. Sequential assessment of hemoglobin/hematocrit and arterial blood gases during therapy for traumatic shock may be useful. These results should be interpreted together in terms of oxygen-carrying capacity and blood-oxygen content. In the trauma patient, the presence of metabolic acidosis in the early phase of resuscitation indicates poor tissue perfusion and should be considered an indicator of inadequate volume resuscitation or ongoing hemorrhage.
Constant reassessment of ventilation and oxygenation, hemodynamic response to volume replacement therapy, and physical examination findings is crucial to detect clinical deterioration, adjust therapy, and identify previously missed injuries. Trauma resuscitations involve multiple individuals performing numerous tasks. During the process of resuscitation, tubes and catheters can become dislodged or disconnected, pneumothoraces can develop or enlarge, external or internal injuries may bleed again as blood pressure increases, pulmonary congestion or contusion may develop in response to volume therapy, core temperature may drop substantially in an exposed patient receiving large amounts of intravenous fluids, or the previously benign abdomen may become distended or rigid. These are examples of significant changes that can occur rapidly during the trauma resuscitation. Careful and constant attention with frequent reassessment is crucial to ensure optimal outcome.
KEY TESTING
• Type and cross
• Base deficit
• Thermoelastography (TEG)
• US of the thorax, pericardium, and abdomen
• Chest x-ray
ED MANAGEMENT
Walter Cannon wrote in 1923, “Since acidosis in shock indicates a deficient delivery of oxygen to active tissues, the rational move is not to treat the effect, but the cause, that is, to provide a better supply of oxygen by early and permanent improvement of the circulation” (6). This approach remains prudent today and is reflected in the ABCs. Initial treatment in traumatic shock occurs concurrently with initial evaluation and is focused on restoring adequate oxygen delivery. Oxygen should be administered to all major trauma patients, regardless of presenting pulse oximetry readings. Early control of the airway in the patient with traumatic shock may be lifesaving and should take priority over all other interventions. Insertion of oral or nasal airways with assisted ventilation and endotracheal intubation are key components of the initial trauma resuscitation.
Gaining intravenous access rapidly is essential to begin volume replacement and support the hemodynamics of the patient in traumatic shock. The antecubital fossa is an excellent site to initiate intravenous access. Large-bore catheters (14 or 16 gauge) and high-flow intravenous tubing should be used to maximize delivery rate of fluids. At least two intravenous sites should be secured. If adequate intravenous access cannot be established within a few minutes, percutaneous femoral venous access, ankle venous cutdown, greater saphenous–venous cutdown at the proximal thigh, subclavian/jugular venous access, or intraosseous access should be considered.
Fluid therapy in trauma is an area of ongoing controversy. Intravascular volume replacement to compensate for blood loss and restore tissue perfusion has been accepted standard therapy for many years. The historical justification for aggressive intravenous fluid resuscitation was to maintain organ perfusion and improve survival. The enthusiasm for the approach was generated from experimental work employing controlled hemorrhage models and for decades, crystalloid loading to normalize blood pressure and heart rate was the standard. However, the Houston randomized clinical trial in 1994, showing no advantage to aggressive preoperative crystalloid resuscitation for penetrating torso wounds and reduced survival for cardiac wounds, prompted a reassessment of the resuscitation of the severely injured trauma patient. This often-cited clinical trial suggested higher survival rates in patients who underwent delayed fluid resuscitation at the time of operative intervention, instead of immediate fluid resuscitation starting in the field, but the benefit was limited to patients with pericardial tamponade (7). Although this trial initiated significant additional research and healthy debate, subsequent clinical trials have not been able to replicate the advantage of hypotensive resuscitation found in the Houston study. Other studies have also suggested that aggressive fluid resuscitation in patients with uncontrolled or ongoing hemorrhage (prior to operative control) may increase blood loss and ultimately mortality. Research has questioned which intervention should take priority, reversal of hypovolemia or control of hemorrhage, when both cannot be achieved simultaneously. Restoring blood volume before active hemorrhage is controlled may result in increased blood loss caused by clot disruption, hemodilution, and loss of clotting factors. Although some studies have criticized aggressive fluid administration as being ineffective (8), others suggest that limited volume replacement, termed hypotensive resuscitation, which maintains minimally adequate organ perfusion, may result in improved outcome (9). The threshold for clot disruption appears to be about 90 mm Hg (10). It is important to remember that most clinical trials evaluating fluid administration have involved penetrating trauma victims. Whether the same principles can be applied to blunt trauma victims is not clear. In fact, this approach may be detrimental to blunt trauma patients with traumatic brain injury (TBI), because hypotension increases mortality in these patients (11). A clear endpoint of fluid therapy, in terms of an optimal blood pressure, has not been defined. Based on the current literature, it appears reasonable to have a goal of a systolic blood pressure of approximately 90 mm Hg for penetrating trauma patients and 100 mm Hg for blunt trauma patients with suspected TBI.
The optimal type of fluid for volume replacement has also been the subject of considerable debate. Crystalloid solutions, such as normal saline or lactated Ringer solution, have long been considered the standard. While hypertonic saline (HTS) is an appealing resuscitative agent, the recent large multicenter Resuscitation Outcomes Consortium (ROC) trial was terminated because of safety issues (12).
Replacing a significant percentage of the patient’s blood volume with fluids that have not been warmed (“room temperature” is significantly cooler than body temperature) can render a patient hypothermic very quickly. Fluids may be warmed ahead of time or be administered through a fluid warmer. In an average-sized adult showing signs of shock, infusion of 2 L of crystalloid and immediate reassessment of hemodynamics is a reasonable course of action (see Chapter 245 for pediatric resuscitation). The point in volume resuscitation at which blood transfusion is initiated has not been well defined. Factors that influence the decision to start blood transfusion include severity of shock, initial response to crystalloid, and anticipated ongoing bleeding. In general, if hemodynamics do not improve after administration of 3 L of crystalloid (or about 50 mL/kg) given rapidly, further volume replacement with blood should be considered. Certain patients may also require transfusion based on their initial injuries, such as those with major pelvic trauma.
The value of colloids in the treatment of traumatic shock remains to be established. Colloids, such as albumin, hetastarch, and dextran, can effectively increase intravascular volume and maintain plasma oncotic pressure at more normal levels compared with crystalloids. However, substantial data demonstrate that crystalloid and colloids are equally effective in resuscitation from hemorrhagic shock (13), and colloids have not been shown to improve outcomes despite higher cost. Furthermore, some evidence suggests that resuscitation with colloids may have deleterious effects. One study found that albumin was associated with significantly worse outcomes in patients with TBI (14) and another trial suggested starches might increase the risk of coagulopathy and AKI (15). Therefore, until well-designed clinical trials are performed showing an advantage to another approach, crystalloids remain the fluid of choice for the resuscitation of trauma patients in hemorrhagic shock (16).
Red blood cell (RBC) substitutes consisting of hemoglobin that has been modified by polymerization and biochemical alteration have been extensively studied in both animal and human trials. Studies have suggested that these RBC substitutes, with their ability to carry oxygen, may be superior to the current, more conventional methods of resuscitation from hemorrhagic shock (17). The USA Multicenter PolyHeme Resuscitation Trial enrolled 720 patients in 32 centers, but the results fail to demonstrate a clinical benefit (18).
Typed and cross-matched PRBCs are the best choice for blood transfusion, although this usually takes at least 30 minutes to obtain. If transfusion is required more urgently, type-specific blood (usually available in 10 to 20 minutes) is an appropriate alternative. Type-specific blood has ABO compatibility with the patient’s blood type but has not been tested for antibody compatibility. If traumatic shock is severe and immediate transfusion is critical, type O Rh-positive blood for males and type O Rh-negative for girls and women of childbearing age should be used until type-specific or typed and cross-matched blood is available. Type O Rh-negative blood should be immediately available in the trauma room, if there is appropriate prehospital notification.
If early blood transfusion is required to manage profound hypovolemia or if there is ongoing massive hemorrhage, fresh-frozen plasma (FFP) and platelets may be needed to restore the coagulation system. If a massive transfusion is anticipated (persistent hypotension with presumed active torso hemorrhage), some clinical studies have suggested a preemptive FFP:RBC ratio of 1:1 although a survival benefit has not been clearly defined. Others have suggested a 1:2 ratio may be more effective (19). A suggested definition of massive transfusion is 10 or more units of RBC in a 12-hour period. Initial large studies have suggested a significant survival benefit, but the role of tranexamic acid (TXA) remains controversial and the clinical benefit has not yet been clearly established (20). Conventional coagulation studies (PRR, INR) and platelet counts generally do not reflect the coagulation status of the critically injured. Point of care measurements, such as thermoelastography (TEG), that allow for early diagnosis of primary fibrinolysis, are now being implemented (21). Recombinant factor VIIa is a hemostatic agent originally developed to treat bleeding in hemophiliacs. Several authors have suggested that factor VIIa may be beneficial in severely bleeding trauma patients. However, two large randomized clinical trials have failed to identify a benefit (22).
Pneumothorax or hemothorax should be managed by the placement of a chest tube (18 French for pneumothorax, 28 French for hemothorax) in the lateral chest with the tube oriented toward the apicoposterior chest wall. While traditionally large chest tubes have been used in these situations, some authors have argued the smaller pigtail catheters (14 French) perform just as well, even for a traumatic hemothorax (23). If a tension pneumothorax is suspected and the patient is hypotensive, the chest tube should be placed immediately even if a drainage system is not available.
If pericardial tamponade is suspected and the patient is hypotensive and worsening despite volume resuscitation, pericardiocentesis is indicated. A pericardiocentesis needle is inserted in the left subxiphoid area and directed 45 degrees toward the left shoulder or sternal notch, whereas suction is maintained through an attached syringe. Although the subxiphoid approach has been described as the “classical” approach for performing pericardiocentesis, the para-apical or parasternal approach with US guidance has been advocated in more recent studies (24). No controlled trials have compared the approaches in trauma patients. If a particular view by US (subxiphoid or parasternal) offers evidence of significant fluid accumulation, it is prudent to take that approach with the pericardiocentesis needle. Blood in the pericardium often forms clots, thus limiting the value of pericardiocentesis, particularly with right ventricular or atrial injuries. If the patient is profoundly hypotensive or has lost a detectable blood pressure in the trauma bay, an emergency left lateral thoracotomy should be performed to open the pericardium. Patients who have not shown signs of life after sustaining blunt trauma are poor candidates for ED thoracotomy (25).
Intra-abdominal injury is a common source for postinjury shock. The clinical presentation and response to initial therapy dictate the subsequent assessment of the abdomen. Hemodynamically unstable patients with physical examination or US evidence of abdominal injury should undergo exploratory laparotomy. Patients with suspected abdominal trauma who have exhibited transient hemodynamic instability should undergo a FAST examination, which can identify free intra-abdominal fluid. DPL is an alternative method of detecting intraperitoneal hemorrhage, if US is not available. Hemodynamically stable patients with potential abdominal trauma are candidates for abdominal CT scanning.
A quick maneuver to treat the hypotensive pregnant trauma patient is to place the patient in the left lateral decubitus position or tilt the backboard up 15 degrees on the right to move the gravid uterus off the inferior vena cava. This positioning improves venous return and may normalize the blood pressure.
In general, vasopressors and inotropic agents are not used in the ED management of traumatic shock except for cases of neurogenic or vasogenic shock (e.g., spinal cord injury) in which peripheral vasodilatation causes or contributes to hemodynamic instability. Agents such as dopamine or norepinephrine may be useful after fluid resuscitation. From the ED perspective, shock rarely occurs from isolated head trauma except in the young child. In cases of myocardial infarction associated with trauma or significant myocardial contusion, inotropic support may be appropriate.
CRITICAL INTERVENTIONS
• Recognize and treat shock aggressively before hypotension develops. Remember that shock may exist in a patient with “normal” vital signs.
• Wrap potentially unstable pelvic fractures with a sheet prior to transfer or extended diagnostic testing.
• When possible, place the hypotensive pregnant trauma patient in the left lateral decubitus position to relieve possible compression of the inferior vena cava.
• Isolated head trauma rarely causes hypotension; search for other causes.
DISPOSITION
Definitive management of the patient with traumatic shock often requires emergency surgery. Consultation with a surgeon should be initiated as soon as possible in all victims of significant trauma who might require operative or critical care interventions. If information from prehospital care providers indicates potentially serious injury, the surgeon (or trauma team) should be notified before the patient arrives in the ED. If transfer to another hospital is required, early mobilization of resources (ambulance, staff, or air medical transport service) should be initiated concurrently with assessment and stabilization.
The criteria for transfer to a trauma center include lack of an experienced trauma surgeon, lack of adequate critical care or support services, and lack of adequate resources to manage a patient’s injuries. The use of scoring systems such as the Trauma Score (TS), Revised Trauma Score (RTS), and the Glasgow Coma Scale (GCS) for triage may be helpful in identifying patients with severe injury. Patients with a TS <12, an RTS of ≤8, or a GCS <10 generally have serious injuries and should be managed within an organized trauma system. The emergency physician must determine which interventions and diagnostic studies are essential before transfer. These decisions are affected by mode of transport, distance to the trauma center, and the capabilities of the referring hospital. For example, delaying transfer to obtain a head and abdominal CT scan or plain radiographs is of no benefit if there is no neurosurgeon, general surgeon, or orthopedist available or if no immediate intervention will take place at the referring hospital. On the other hand, if a head, chest, or abdominal CT scan is indicated and can be obtained while waiting for the ambulance or helicopter to arrive (and will not cause a delay in transfer), it is best to proceed with these studies.
Common Pitfalls
• Failure to recognize shock; normal vital signs can be misleading.
• Failure to realize that altered mental status is a sign of shock, particularly when there is no evidence of head trauma.
• Failure to monitor trauma patients carefully and to perform serial examinations to detect changes in clinical status and response to interventions. An initial favorable response to volume replacement should not mislead the physician to be less vigilant, as adequate initial resuscitation can temporarily mask significant ongoing hemorrhage.
• Delaying surgical consultation or transfer to a trauma center.
• Missing subtle injuries, especially in patients with severe multisystem trauma.
REFERENCES
1. American College of Surgeons Committee on Trauma. Advanced Trauma Life Support: Providers’ Manual. Chicago, IL: American College of Surgeons; 2004.
2. Davis JW, Kaups KL. Base deficit in the elderly: A marker of severe injury and death. J Trauma. 1998;45(5):873–877.
3. Kamolz LP, Andel H, Schramm W, et al. Lactate: Early predictor of morbidity and mortality in patients with severe burns. Burns. 2005;31:986–990.
4. Sauaia A, Moore FA, Moore EE, et al. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45:291–301.
5. Griffen MM, Frykberg ER, Kerwin AJ, et al. Radiographic clearance of blunt cervical spine injury: Plain radiograph or computed tomography scan? J Trauma. 2003;55:222–226.
6. Cannon WB. Traumatic Shock. New York, NY: Appleton and Co; 1923.
7. Bickell WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109.
8. Roberts I, Evans P, Bunn F, et al. Is the normalisation of blood pressure in bleeding trauma patients harmful? Lancet. 2001;357:385–387.
9. Stern SA, Dronen SC, Birrer P, et al. The effect of blood pressure on hemorrhage volume and survival in a near-fatal hemorrhage model incorporating a vascular injury. Ann Emerg Med. 1993;22:155–163.
10. Sondeen JL, Coopes VG, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003;54: S110–S117.
11. Winchell RJ, Simons RK, Hoyt DB. Transient systolic hypotension: A serious problem in the management of head injury. Arch Surg. 1996;131(5):533–539.
12. Bulger EM, May S, Kerby JD, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock. A randomized, placebo controlled trial. Ann Surg. 2011;253:431–441.
13. Shierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: A systematic review of randomised trials. BMJ. 1998;316:961–964.
14. SAFE Study Investigators. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884.
15. Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation. A systematic review and meta-analysis. JAMA. 2013;309:1229.
16. Rizoli SB. Crystalloids and colloids in trauma resuscitation: A brief overview of the current debate. J Trauma. 2003;54:S82–S88.
17. Gould SA, Moore EE, Hoyt DB, et al. The life sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195(4):445–452.
18. Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: The USA multicenter trial. J Am Coll Surg. 2009;208:1–13.
19. Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: Is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270.
20. Napolitan LM, Cohen MJ, Cotton BA, et al. Tranexamic acid in trauma: How should we use it? J Trauma Acute Care Surg. 2013;74:1575–1586.
21. Kashuk JL, Moore EE, Sawyer M, et al. Primary fibrinolysis is integral in the pathogenesis of acute coagulopathy of trauma. Ann Surg. 2010;252:434–442.
22. Hauser CJ, Boffard K, Dutton R, et al. Results of the CONTROL trial: Efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma.2010;69:489–500.
23. Kulvatunyou N, Joseph B, Friese RS, et al. 14 French pigtail catheters placed by surgeons to drain blood on trauma patients: Is 14-Fr too small? J Trauma Acute Care Surg. 2012;6:1423–1427.
24. Tsang TS, Enriquez-Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429–436.
25. Branney SW, Moore EE, Feldhaus KM, et al. Critical analysis of two decades of experience with postinjury emergency department thoracotomy in a regional trauma center. J Trauma. 1998;45:87–94.